Equation 4
This equation will allow you to calculate the concentration of salt that has the same Relative Humidity (RH) in equilibrium with a PEG or other solute solution. You will need to provide the molecular weight of the PEG in Da, the ionisation state (how many species it dissociates into) of the salt you would like to use and a term describing the the specific volume of the salt (for sodium chloride y = 0.027, for ammonium sulfate y = 0.074, for sodium acetate y = 0.054, for sodium malonate y = 0.095, for magnesium sulphate y = 0.045, for Monopotassium phosphate y = 0.058 and for Dipotassium phosphate y = 0.071).
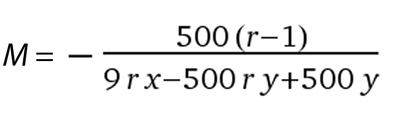
The Relative Humidity (RH - r in the equation above) in equilibrium with solutions can be understood in terms of Raoult's law. It has two aspects that are counter-intuitive and lead to some surprising observations. The first is that the number of equivalent molecules in solution must be accounted for. This means that for sodium chloride, each ion in solution counts as a molecular equivalent. This requires knowledge of the ionization behaviour of the substance in solution. For example, ammonium sulfate effectively dissociates into two ions [NH4+ and (NH4SO4)-] and not three as might be expected. Raoult's law starts to break down for PEG solutions over a molecular weight of 1000 Da but this can be corrected using the Flory-Huggins model for the entropy of mixing (used in equation 2).
y can be calculated for other salts using the following formula: y = [(1/ρ)M]/1000



