- Home
- Industry
- Industry news
- Controlling diabetes...
Controlling diabetes through increased peroxisome proliferator-activated receptor activity
11-09-2009
Sanofi-Aventis design better drugs for the treatment of diabetes thanks to their use of the ESRF MXpress service. They used synchrotron light to study macromolecular crystals of nuclear receptor proteins with bound agonists.
Share
What was the challenge?
Type 2 diabetes is caused by the body’s ineffective use or resistance to insulin. It is often termed late-onset diabetes and can develop in adults as a result of poor diet and lack of exercise. The number of cases of type 2 diabetes is on the increase in the developed world. If left untreated, as with other complex metabolic complaints, type-2 diabetes can lead to organ damage and in particular damage to the cardiovascular system.
Background
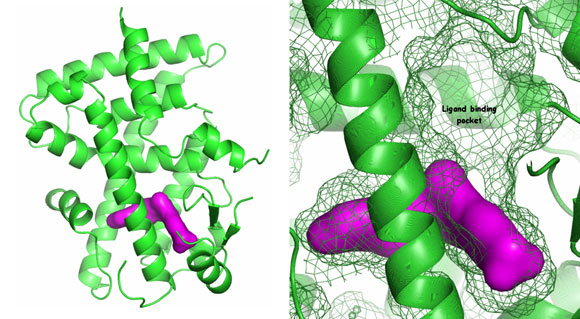
The response of the body to insulin I is regulated by nuclear receptor proteins called peroxisome proliferator-activated receptors, PPAR. Increased PPARδ activity is considered to have a positive effect on type 2 diabetes. The crystal structures of the ligand binding domains of the PPARδ receptor in complex with agonist molecules were studied in order to find an agonist which could be used to stimulate the body's reaction to insulin in sick patients.
Synchrotron techniques
Macromolecular crystallography, MXpress service.
Results
It was already known from published structures that the binding pocket was large. Structures with Sanofi-Aventis in-house agonists showed the high degree of plasticity of this pocket – a key discovery for the chemists trying to develop drugs. The analysis of the binding mode of the ligands allowed their optimisation in order to activate PPARδ with high specificity, taking advantage of the properties of the ligand binding pocket. Currently Sanofi-Aventis has a new drug in clinical trials as part of their diabetes portfolio.
How did the synchrotron experiment help?
PPARδ crystals are slow to grow, diffract weakly (in a monoclinic spacegroup) and are sensitive to X-rays. These crystals did not diffract sufficiently in our in-house source, and were damaged rapidly under synchrotron light. Usable data could only be collected at the synchrotron on “soft” beamlines.
Top image: PPARδ ligand binding domain