- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2011
- Structure of materials
- When oil and water do mix: the nanoscale structure of a surfactant-modified hydrophobic interface
When oil and water do mix: the nanoscale structure of a surfactant-modified hydrophobic interface
Hydrophobicity plays a dominant role in fields ranging from the structure of living matter, like cell membrane stabilisation and protein folding, to microemulsion-mediated nano-particle formation [1]. Surfactants can be used to reduce the hydrophobic barrier between oil and water as demonstrated by the use for millennia of detergent-containing water to solubilise and remove oils from fabrics, crockery and the human body. Nevertheless, until recently, the very challenging experiments required have prevented a molecular-resolution determination of the structure of the prime surfactant-modified hydrophobic interface, that between oil and water. Thus, a key ingredient in the fundamental understanding of the relation between surfactants and the hydrophobic interaction was still missing.
Using X-ray reflectivity at the high-energy microfocus beamline ID15A and surface tensiometry, the molecular-scale structure and thermodynamics of deeply buried oil/water interfaces decorated by ionic surfactants have been measured for the first time.
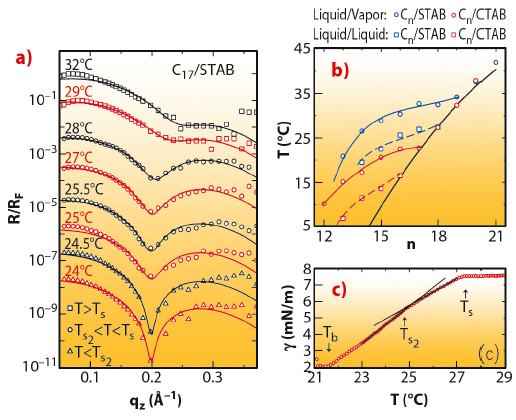
The interface between bulk oil (alkane) and pure water is simple, showing a monotonic transition from the density of oil to that of water over a distance of a few angstroms, determined by the capillary waves decorating all liquid interfaces [2]. When the pure water phase is replaced by a very dilute (sub-millimolar) water solution of an alkyl ammonium bromide cationic surfactant, an intriguingly complex interfacial structure is found. At high temperatures, a liquid-like layer consisting of a mixture of alkane molecules and surfactant tails is found to intrude between the alkane and solution bulk phases. This is manifested by the appearance of a shallow dip in the X-ray reflectivity (Figure 44a). The layer has a thickness smaller than the length of a single extended alkane molecule. Upon cooling this layer undergoes an abrupt freezing transition at Ts to a solid monolayer of densely-packed, fully-extended, interface-normal alkane molecules. This transition is observed in Figure 44a as a sharpening of the dip at Ts2 ~28°C and its shifting to a lower scattering vector qz. Moreover, about 3°C below this transition, a second transition is observed, manifested in an abrupt sharpening of the dip, but no shift in its position. This is a transition from a rotator to a fully crystalline monolayer, as demonstrated also by the magnitude of the entropy loss at the transition, measured from the slope change occurring at the transition in the linear temperature dependence of the surface tension (Figure 44c).
 |
|
Fig. 44: a) Measured (symbols) and modelled (lines) Fresnel-normalised X-ray reflectivity of the interface between a bulk liquid of 17-carbon alkane and a 0.16 mM aqueous STAB surfactant solution. b) Measured (symbols) and modelled (lines) interfacial freezing temperatures Ts(n) of alkane monolayers at the liquid/vapour (solid lines) and liquid/liquid (dashed lines) interfaces of 0.6 mM CTAB (red) and 0.16 mM STAB (blue) solutions, and the alkane’s bulk freezing temperatures Tb (black line). c) Temperature dependence of the surface tension for the interface in (a). Arrows mark the transitions. |
The temperature-dependent surface tension measurements, carried out for a range of alkane lengths for two different-length but same headgroup surfactants (CTAB and STAB), determined the phase diagram of the interface, shown in Figure 44b. An increasing temperature range of existence is found for the interface-frozen monolayer as the alkane length (n) decreases, reaching tens of degrees at low n. The phase boundaries, Ts(n), are similar to, but downshifted from, those observed for alkane monolayers spread on the liquid/vapour interface of the same solutions for both surfactants [3]. The study offers a Mixture Theory based thermodynamic model for the transition, dominated by the interchange energy cost of replacing a surfactant tail with an alkane molecule. This model reproduces faithfully Ts(n) at both the bulk alkane/solution and solution/vapour interfaces (Figure 44b). The phase sequence and transitions are summarised pictorially in Figure 45.
 |
|
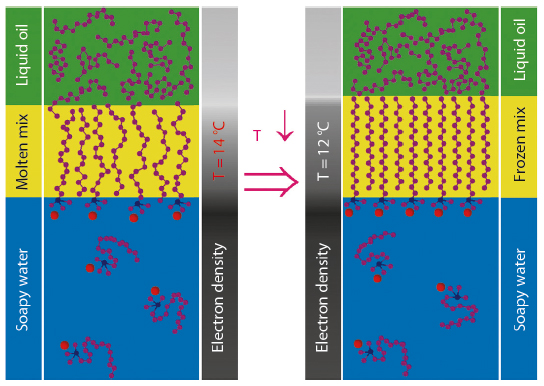
Fig. 45: Pictorial summary of the interfacial phase transition for an aqueous CTAB solution/15-carbon liquid alkane interface. At 14°C, the oil and surfactant molecules intermix at the interface forming a single monolayer of flexible, kinked, and partly aligned chains. At 12°C, the chains abruptly assume a fully stretched conformation, thus yielding a frozen, solid, two-dimensional interfacial monolayer, while the overlying alkane phase remains liquid. |
Similar studies on other surfactants should reveal whether the behaviour found here is typical, and will allow a deeper insight into the microscopic origins of hydrophobicity.
Principal publication and authors
L. Tamam (a), D. Pontoni (b), Z. Sapir (a), S. Yefet (a), E. Sloutskin (a), B. Ocko (c), H. Reichert (b) and M. Deutsch (a), Proc. Nat. Acad. Sci. USA 108, 5522-5525 (2011).
(a) Physics Department and Institute of Nanotechnology, Bar-Ilan University, Ramat-Gan (Israel)
(b) ESRF
(c) Soft Condensed Matter & Materials Science Department, Brookhaven National Laboratory, Upton NY (USA)
References
[1] D. Chandler, Nature 437, 640 (2005).
[2] B.M. Ocko et al., Phys. Rev. Lett. 72, 242 (1994).
[3] E. Sloutskin et al., Phys. Rev. Lett. 99, 0136102 (2007).



