- Home
- News
- Spotlight on Science
- Gold in high-temperature...
Gold in high-temperature ore-forming fluids has a ubiquitous complex
26-06-2019
A state-of-the-art experimental study complemented by advanced computer modeling reveals that the chemistry of Au in high-temperature hydrothermal fluids of highly-variable composition is controlled by a simple gold(I) chloride complex. Geometrical flexibility explains the predominance of this complex in extremely diverse fluids.
Chloride-rich fluids are widespread in the Earth’s interior from the low-temperature subsurface environment to high-pressure high-temperature conditions of the deep lithosphere. The concentration of chloride salts in hydrothermal ore-forming fluids can be highly variable, from diluted aqueous solutions to concentrated chloride brines and anhydrous (dry) chloride melts beneath volcanoes. These fluids accumulate and transport metals and form deposits of metallic minerals in the Earth’s crust. World-class gold deposits are most commonly associated with hydrothermal ore-forming systems.
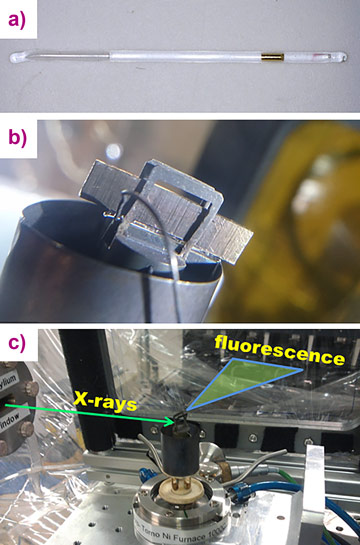
New in situ X-ray absorption spectroscopy data combined with ab initio molecular dynamics (AIMD) simulations has revealed that the hydrothermal chemistry of ore metals is controlled by simple aqueous species in a wide range of T-P-compositional parameters. In particular, the transport of Au in high-temperature fluids can be explicitly described by the formation of AuCl2- at any salt concentration from low-salinity fluids to hydrosaline brines and anhydrous melts. To investigate the state of Au at high temperatures and pressures, we recorded Au L3-edge X-ray absorption spectra of aqueous chloride-bearing fluids at temperatures 350 – 575°C and pressures up to 4500 bar. The composition of the experimental fluids varied from HCl-dominated solutions to concentrated NaCl, KCl, and CsCl brines. X-ray absorption spectra of dry NaCl / KCl / CsCl melt were recorded at 650°C. For the experiments, a capillary technique was adopted whereby the experimental fluid was loaded inside a silica glass capillary together with a small piece of Au (Figure 1). The spectra were registered after the fluid was saturated with Au at the experimental parameters. Two series of XAS experiments were performed: total fluorescence yield (TFY) detection at the BM20 beamline, and high energy resolution fluorescence detection (HERFD) at beamline ID26.
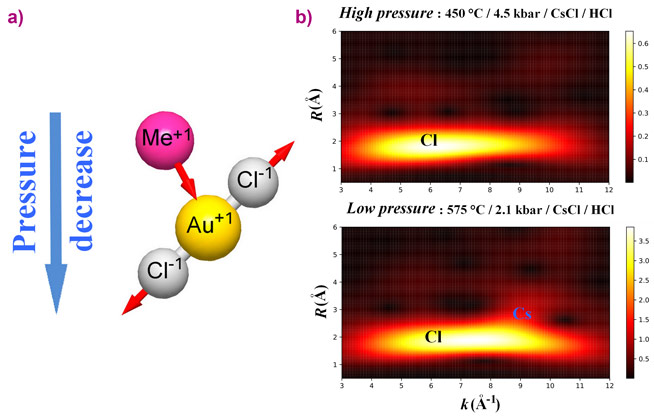
Interpretation of TFY-EXAFS spectra showed that, independently of the experimental system composition, Au was coordinated by two Cl atoms (RAu-Cl = 2.25 – 2.28 Å) in the first coordination sphere, while 0.2 – 0.6 alkali metal atoms were detected in the second coordination sphere at RAu-Me = 3.3 – 4.1 Å. The presence of the alkali metal atoms in the vicinity of the Au-Cl complex was confirmed by the HERFD-XANES spectroscopy: the white line intensity decreased substantially in NaCl-dominated fluid and dry melt in comparison with HCl-dominated fluid. According to the AIMD simulations followed by the calculation of atomic charges and theoretical XANES spectra modeling, the observed decrease of the white line intensity stems from the partial compensation of the positive charge located on Au due to the presence of the distant-coordination-sphere alkali metal cations.
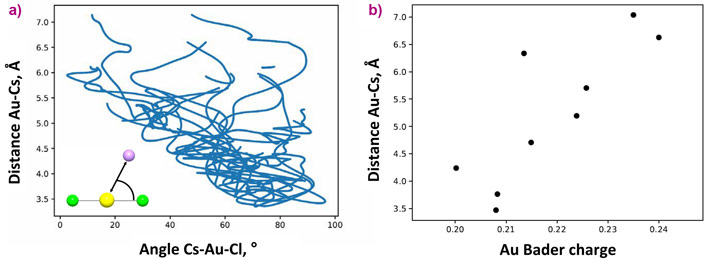
The main theme of this study concerns the topology (flexibility of the chemical bonds in terms of interatomic distances and angles) of the Au-Cl complex as a function of the system parameters and composition. The interatomic distances between Au and atoms localised in the first and second coordination spheres exhibit contrasting behavior with respect to fluid pressure and composition. A decrease of the fluid pressure (or dielectric constant) promotes ionic interaction between AuCl2- and the distant-coordination-sphere cations and causes second-sphere compression (Figure 2). Contraction of the distance between Au and the cations in the second sphere results in partial compensation of the positive charge located on Au (Figure 3). A decrease of the Au charge weakens the interaction between Au+ and the nearest Cl- ligands. As a result, the Au-Cl distances slightly increase. A similar effect is caused by the increase of the number of the cations in the vicinity of Au in dry chloride melt and concentrated chloride brines. Accordingly, the bonds are to some extent flexible despite the mostly covalent character of strong chemical bonds between Au and the nearest Cl ligands.
These results demonstrate that the chemistry of ore-forming fluids exhibits a tendency of simplification with an increase of the temperature. In the case of Au, the predominance of AuCl2- increases due to the flexibility of chemical bonds in the first and second coordination spheres of Au. As a result, the AuCl2- complex is the main species in high-temperature fluids. In low-temperature sulfur-bearing fluids (< 350°C), the hydrothermal transport of Au is mostly accounted for by the formation of the hydrosulfide complex Au(HS)2- independently of the fluid composition and redox state [1]. This behavior is valid not only for Au but for other ore metals. For example, in the case of Pt, the PtCl42- complex predominates in high-temperature hydrothermal fluids [2]. The simple speciation scheme described here enables the development of robust but simple thermodynamic models to describe the hydrothermal transport of metals in a wide range of physico-chemical parameters. In thermodynamic calculations, the effect of dissolved chloride salts on the solubility of ore minerals can be explicitly accounted for by the activity coefficients of simple aqueous complexes calculated by means of the extended Debye-Hückel equation even in the case of high-temperature concentrated brines.
Principal publication and authors
Gold transport in hydrothermal chloride-bearing fluids: insights from in situ X-ray absorption spectroscopy and ab initio molecular dynamics, B.R. Tagirov (a), A.L. Trigub (b), O.N. Filimonova (a), K.O. Kvashnina (c), M.S. Nickolsky (a), S. Lafuerza (c) and D.A. Chareev (d), ACS Earth and Space Chemistry 3, 240-261 (2019); doi: 10.1021/acsearthspacechem.8b00103.
(a) Institute of Geology of Ore Deposits, Petrography, Mineralogy and Geochemistry, Moscow (Russia)
(b) National Research Centre ‘Kurchatov Institute’, Moscow (Russia)
(c) ESRF
(d) Institute of Experimental Mineralogy, Chernogolovka (Russia)
References
[1] A.L. Trigub et al., Chem. Geol. 471, 52-64 (2017); doi: 10.1016/j.chemgeo.2017.09.010.
[2] B.R. Tagirov et al., Geochim. Cosmochim. Acta 254, 86-101 (2019); doi: 10.1016/j.gca.2019.03.023.