- Home
- News
- Spotlight on Science
- Reentrant behaviour...
Reentrant behaviour of a solution undergoing inverse melting
29-01-2007
Using X-ray diffraction and differential scanning calorimetry methods, the phase diagram of a solution undergoing inverse melting has been determined. Two different disordered fluid phases separated by a solid region have been observed in the "high concentration" range. At decreasing concentrations, the solid phase fades out and the experimental observations become compatible with a direct transition between a low temperature liquid and a high temperature liquid.
The phenomenon of inverse melting, widely studied in recent years [1-2], happens when a liquid heated at constant pressure undergoes a reversible liquid-to-crystal transition. Such a transition of endothermic nature implies the passage from a low-temperature liquid (LTL) to a high-temperature crystal (HTC) with absorption of heat. This phenomenology, opposite to what is expected for common liquids, indicates the presence of a crystal with entropy higher than its liquid counterpart. If further heated the HTC may eventually melt into a HTL.
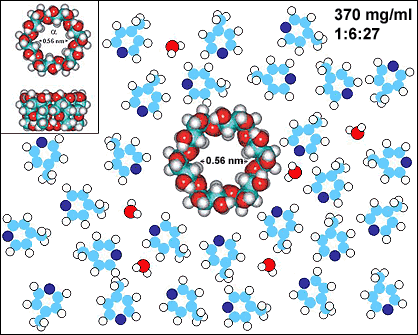
Differential scanning calorimetry (DSC) measurements performed at the University "La Sapienza" in Rome and X-ray diffraction performed at the ESRF beamline BM29 have been carried out on a solution which shows inverse melting: α-cyclodextrin (αCD) (C36H60O30), water and 4-methyl-piridyne (4MP) (C6H7N) [3]. Figure 1 contains a sketch of a solution of αCD-water-4MP.
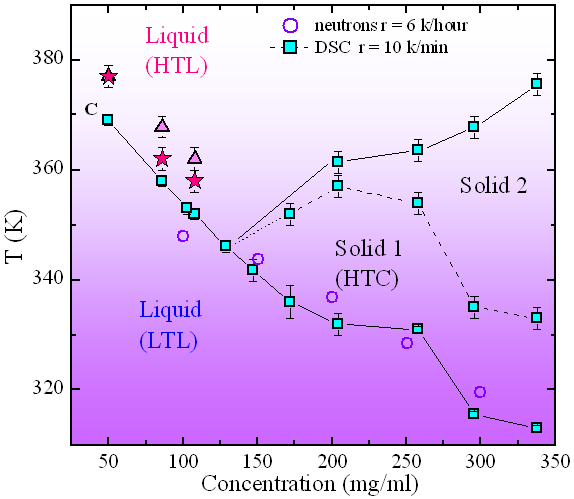
Thanks to the DSC measurements, the inverse phase transitions have been determined and characterised from an energetic point of view. The measurements of heat absorption as a function of the temperature show peaks of endothermic nature, the temperatures at which each transition takes place are reported as full squares in Figure 2. In the concentration region 150-350 mg/ml of αCD in 4MP, three endothermic transitions are observed with increasing temperature: the first corresponds to the LTL-HTC, the second has been attributed to a solid-solid transition, and the third corresponds to the HTC-HTL transition. At decreasing αCD concentration, in the region 50-150 mg/ml, the solid region disappears and a single endothermic transition peak is observed. This experimental observation is compatible with a direct LTL-HTL transition.
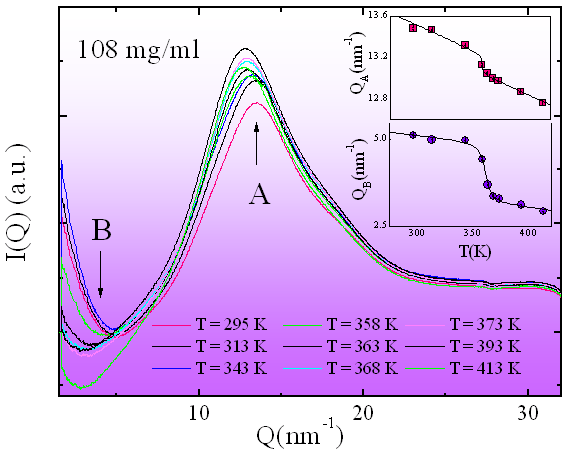
In order to give a microscopic interpretation of the behaviour of the liquid and to investigate its local structure in the “low concentration” region, the X-ray diffraction experiment has been performed for various temperature values along the LTL-HTL transition. Three different concentrations of αCD in 4MP have been investigated. An example of the integrated scattered intensity I(Q) at the concentration 108 mg/ml and in the temperature range 295 - 413K is reported in Figure 3.
Figure 3 confirms the existence of a disordered structure both below and above the transition temperature as detected by the DSC experiment. The temperature evolution of the structure of the liquid has been followed looking at the Q position of the first maximum (around Q=13 nm-1) and minimum (around Q=4 nm-1) of I(Q) respectively identified as positions QA and QB.The Q values of these two points, reported as a function of temperature in the insets of Figure 3, show a jump at the temperatures corresponding to the LTL-HTL transition, as derived by the DSC measurements. The three transition temperatures for the maxima (full triangle) and the minima (full stars) of I(Q) reported on the phase diagram of Figure 2 show good agreement.
The combination of DSC and X-ray diffraction allowed us to draw the phase diagram of a solution undergoing inverse melting and to characterise the two different microscopic local structures of the disordered fluid phases, the LTL and the HTL observed in the low concentration region.
References
[1] A. Lindsay Greer, Too hot to melt, Nature 404, 134 (2000).
[2] A. Crisanti and L. Leuzzi, Phys. Rev. Lett. 95, 087201 (2005) and references within.
[3] M. Plazanet, C. Floare, M.R. Johnson, R. Schweins, and H.P. Trommsdroff, J. Chem. Phys. 121, 5031 (2004).
Authors
R. Angelini (a), G. Ruocco (a,b), S. De Panfilis (c), F. Sette (c), submitted.
(a) CRS SOFT- INFM-CNR, Università "La Sapienza", Roma ( Italy)
(b) Dipartimento di Fisica, Università "La Sapienza", Roma (Italy)
(c) ESRF