- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2017
- Structure of materials
- Probing mechanochemistry with in situ real-time X-ray powder diffraction
Probing mechanochemistry with in situ real-time X-ray powder diffraction
X-ray powder diffraction was used for real-time in situ monitoring of mechanochemical co-crystallisation. For the first time, the macroscopic dynamics of the powder was isolated from the chemical kinetics of the transformation. This revealed unprecedented time-dependent shifts in the kinetics of the process and rapid loss of free-flowing powder.
The use of mechanical force to induce chemical and physical transformations, i.e. mechanochemistry, was first used by early man, who mechanically ruptured covalent bonds to produce fire. Today, mechanochemistry is widespread, adopted by crystal engineers, physicists, materials scientists, chemists, pharmacists and industrialists. A particularly promising aspect of mechanochemistry is its ability to produce novel materials and compounds with high yields and very low environmental impact, i.e. solvent-free processing.
Despite the broad applications of mechanochemistry, the mechanisms that drive these processes are only poorly understood. In fact, recent work has demonstrated that different types of mechanical treatment yield different products [1], and that unique reacting zones can exist even in the same milling jar [2,3]. This lack of control and understanding is restrictive for the further development of mechanochemical technologies in industry, where formation of undesirable and unknown products can have considerable legal and safety repercussions.
To probe the dynamics of mechanochemical co-crystallisation, a simple model system (glycine [gly] and oxalic acid dihydrate [OAD]) was studied by real-time, in situ X-ray powder diffraction (XRPD) at beamline ID11. The system represents a common situation in organic mechanochemistry, where one of the starting materials is present as a hydrate or solvate. In such situations, an additional liquid is often not required to help facilitate the reaction, greatly simplifying the system under investigation. This system was of further interest as it forms two competing products: glycinium semi-oxalate [GO] and bis(glycinium) oxalate [G2O].
A new methodology was developed to process the in situ XRPD patterns that were collected during the experiment. In doing so, this was the first time that critical information was obtained regarding the free-flowing powder within a milling jar. Surprisingly, powder disappeared exponentially from the path of the X-ray beam. This finding is critical to the reliability of in situ XRPD techniques to probe the mechanisms of mechanochemistry. The loss of powder from the beam path indicates caking of material within the ball mill, leading to a fundamental change in the composition and rheology of the system under investigation. By subsequent ex situ analysis, it was found that the ratio of the competing products was different in the free-flowing powder and the powder caked to the ends of the vessel. In situ, real-time analysis did not reflect the true situation occurring in the ball mill. A similar situation has been demonstrated by ex situ analysis [2].
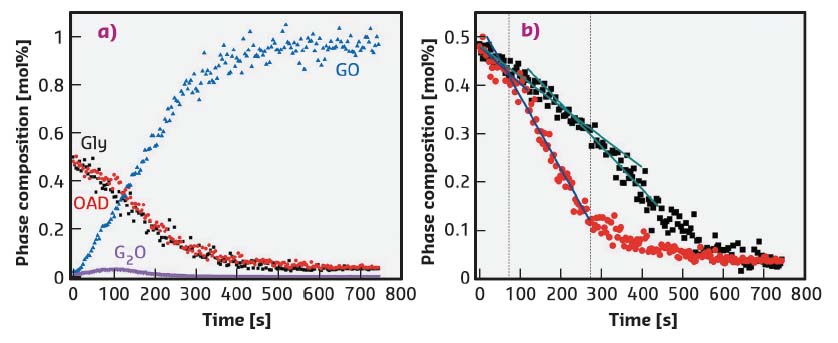
The loss of free-flowing powder also leads to a convolution of macroscopic dynamics and the kinetics of chemical conversion. In these experiments, deconvolution of these functions revealed a linear reaction profile, shown in Figure 121. In situ XRPD is incapable of resolving molecular-level mechanisms, the kinetics of which are surely not first order. However, this linear profile suggests that mixing plays a negligible role in determining reaction progress. Modelling of reaction progress in such cases should be trivial, and is promising for industrial applications of mechanochemistry. Despite its apparent initial simplicity, a sudden and unexpected shift in the rate of the reaction was observed as milling progressed (Figure 121). This unprecedented phenomenon led to a doubling of reaction rates in some cases. The rate increase was most obvious in the rate of consumption of OAD, and likely reflects a decrease in OAD particle size, known to hasten the release of water [4].
 |
|
Fig. 121: Phase composition profile for the milling reaction of gly. and OAD. a) Complete phase profiles for milling reaction at 30 Hz. b) Consumption of OAD at milling frequencies 30 Hz (red) and 25 Hz (black). Change in kinetic profile is denoted by a dotted line in each case. |
The study of mechanochemical processes is vital to the development of enhanced industrial technologies. However, without deeper understanding and control, mechanochemical techniques remain under-exploited. While in situ XRPD approaches are promising to investigate mechanochemical processes in real time, they remain limited by the quantity of sample that they probe. These techniques are further restricted by the level of molecular detail they offer, being restricted to the macroscopic study of kinetics and bulk composition. Despite its limitations, in situ real-time investigations of mechanochemical processes by XRPD remain a powerful means to obtain time-resolved information, such as dramatic shifts in kinetics, that are otherwise not observable.
Principal publication and authors
Challenges of mechanochemistry: is in situ real-time quantitative phase analysis always reliable? A case study of organic salt formation, A.A.L. Michalchuk (a, b, c), I.A. Tumanov (a, d), S. Konar (b), S.A.J. Kimber (e), C.R. Pulham (b, c) and E.V. Boldyreva (a, d), Adv. Sci. 9, 1700132 (2017); doi: 10.1002/advs.201700132.
(a) REC-008 Novosibirsk State University (Russian Federation)
(b) EaStChem School of Chemistry and Centre for Science at Extreme Conditions (CSEC), University of Edinburgh (UK)
(c) EPSRC Centre for Continuous Manufacturing and Crystallisation (CMAC) (UK)
(d) Institute of Solid State Chemistry and Mechanochemistry SB RAS, Novosibirsk (Russian Federation)
(e) ESRF
References
[1] I.A. Tumanov et al., Dokl. Chem. 457, 154-159 (2014).
[2] A.A.L. Michalchuk et al., CrystEngComm 15, 6403-6412 (2013).
[3] A.A.L. Michalchuk et al., Faraday Discuss. 170, 311-335 (2014).
[4] H. Tanaka, J. Therm. Anal. 29, 1115-1122 (1984).



