- Home
- News
- Spotlight on Science
- Chemistry under...
Chemistry under sheer force
02-03-2023
Using high-pressure X-ray diffraction experiments at beamline ID15B, researchers demonstrated that extreme pressure can destroy the ionic bonds in silver iodide, transforming it to elemental silver and iodine. The ability to change the chemistry of materials with mechanical force is an ideal approach for the synthesis of new materials.
The use of mechanical force to prompt chemical reactions is well known to us. In 1820, when Michael Faraday used trituration (grinding) in a mortar to induce the mechanical reduction of silver chloride with zinc, tin, iron and copper, he gave us probably the first experimental example of mechanochemistry. Mechanochemistry directly converts mechanical energy to chemical energy, or chemical potential. Mechanical milling is the most common way of performing mechanochemistry, but the force applied is limited, so many materials are still chemically stable under such relatively gentle pressures. But what happens if the applied pressure is high enough? How can we better understand ionic bonding at the critical point of chemical reaction?
In this work, the mechanochemistry of silver iodide (AgI) was studied at extremely high pressures. At relatively low pressures, AgI exists in various solid phases. At pressures above about 0.5 GPa, AgI has an ionic structure like rock salt and, above about 10 GPa, it transitions to a ‘superionic’ solid. In its superionic phase, the iodine atoms behave like a liquid, flowing through the silver atoms, which remain solid. This exotic phase makes it a promising candidate for making battery electrolytes.
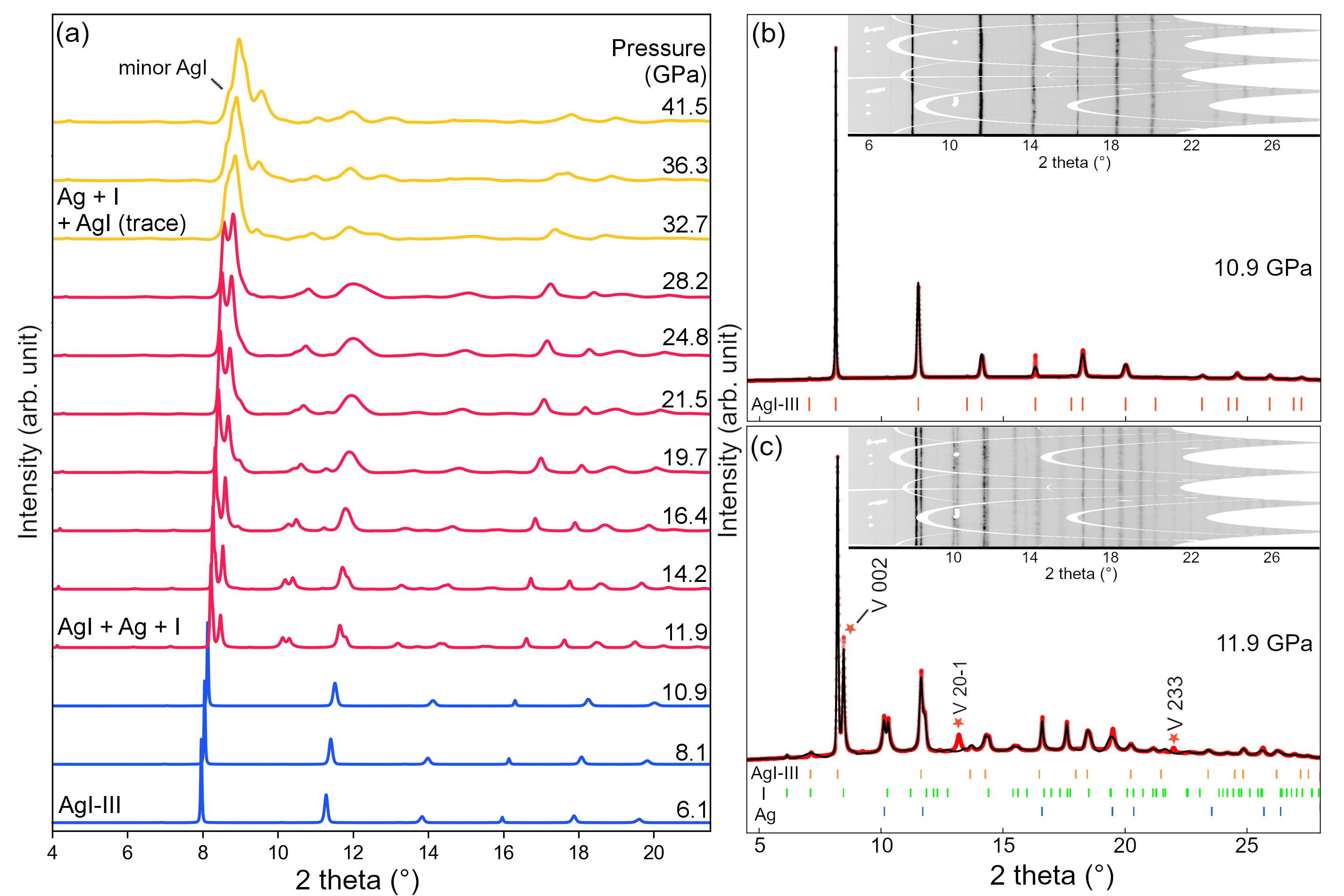
A high-pressure experiment was performed at beamline ID15B, where a pair of diamond anvils was used to compress a tiny chip of silver iodide (AgI) to extremely high pressures, equivalent to 420,000 atmospheres, and high-energy, angular-dispersive X-ray diffraction (XRD) experiments were carried out to measure the structure of the AgI sample under such pressurised conditions. Along the pressurisation trajectory, the disappearance of solid AgI and the emergence of elemental Ag and I was clearly observed in the diffraction patterns (Figure 1). The data show that the external mechanical stress weakens the Ag-I ionic bonds, making AgI chemically unstable and, once the chemical limit of AgI is reached and overcome by increasing pressure, the sample decomposes into elementary silver and iodine due to the collapse of ionicity. Each bond is seen to have its own chemical limit.
Click image to enlarge
Fig. 1: X-ray diffraction (XRD) patterns of compressed AgI. a) Evolution of XRD patterns from 6.1 to 41.5 GPa. b) FCC AgI-III at 10.9 GPa. c) Mixture of AgI, Ag, and I at 11.9 GPa. AgI sample partially decomposes and the peaks of FCC-Ag and Immm-type I appear. Inset figures in (b) and (c) are caked two-dimensional diffraction patterns, whose y-axes are the azimuth angle.
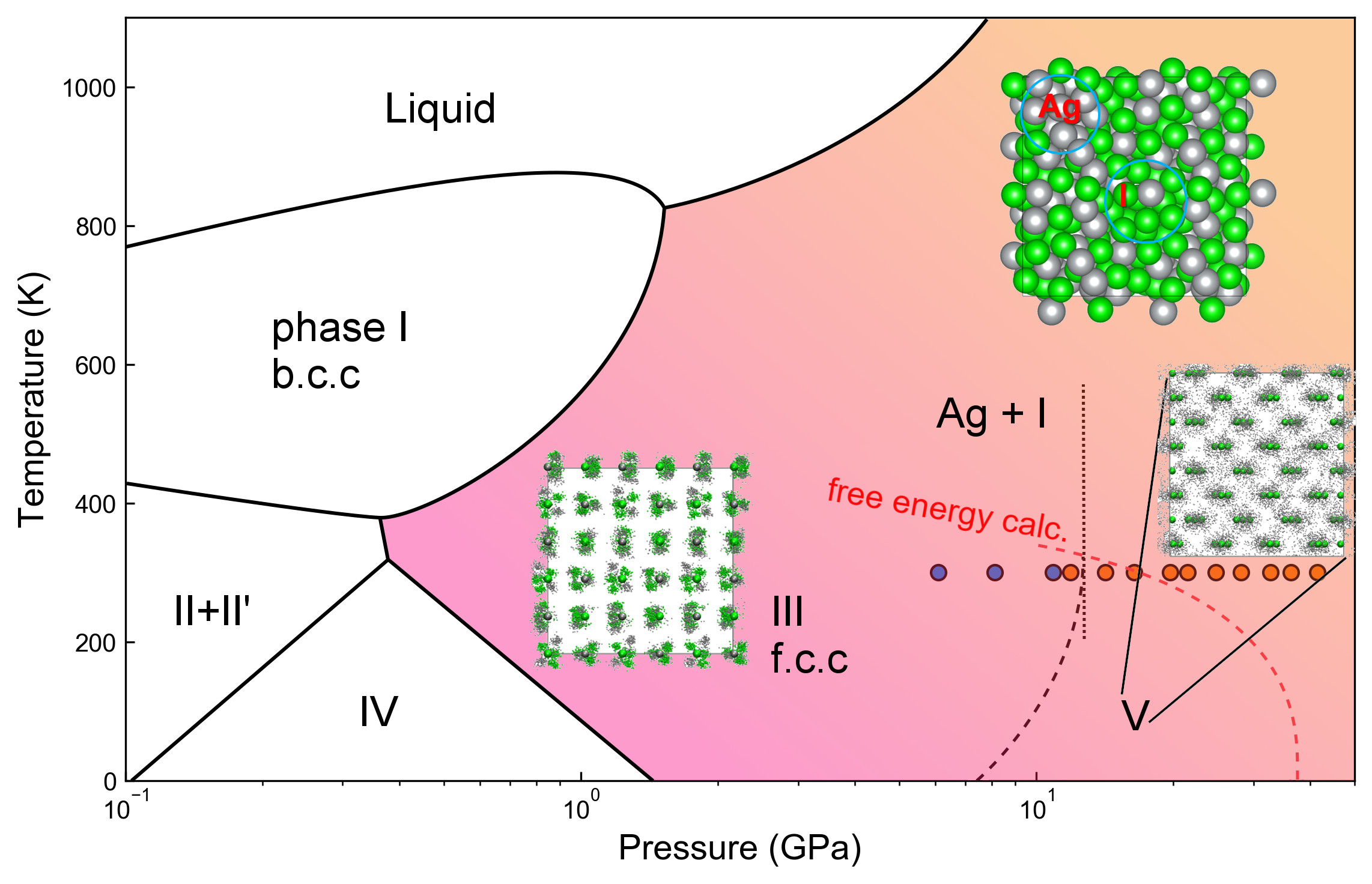
This experiment was pioneered by state-of-the-art computational modelling, in which the evolution of Ag-I bonding and its properties were calculated at high-pressures. Using a structural searching algorithm implemented by the CALYPSO (crystal structure analysis by particle-swarm optimisation) software program, it was possible to derive the ground state of compounds at relevant pressures. Calculating the free energy of Ag, I, and the stable AgI phases gave a predicted decomposition pressure, which was consistent with experimental data. The phase diagram of AgI was thus extended and updated, as shown in Figure 2. Computer modelling further predicts that such pressure-induced chemistry should also occur in other ionic solids such as silver chloride and silver bromide, but at even higher pressures.
Click image to enlarge
Fig. 2: Phase diagram of the Ag−I compound. Inset figures are the trajectories of the supercell structure taken from molecular dynamics (MD) simulation, with ordering solid in AgI-III, superionic solid in AgI-V and decomposed AgI at the end of MD simulation.
In conclusion, the results reveal the sophisticated chemistry of simple inorganic compounds under extreme conditions, and the combined computational and experimental approach may aid in the design of new paths for chemical reactions.
Principal publication and authors
Mechanochemistry and the evolution of ionic bonds in dense silver iodide, J. Li (a), Y. Geng (a), Z. Xu (a), P. Zhang (b), G. Garbarino (c), M. Miao (d), Q. Hu (e), X. Wang (a), JACS Au. 3, 2, 402-408 (2023); https://doi.org/10.1021/jacsau.2c00550
(a) School of Physics and Electronic Information, Yantai University, Yantai (China)
(b) School of Physics and Electronic Engineering, Linyi University, Linyi (China)
(c) ESRF
(d) Department of Chemistry and Biochemistry, California State University, Northridge, California (USA)
(e) Center for High Pressure Science and Technology Advanced Research, Beijing (China)
| About the beamline: ID15B |
|
Beamline ID15B is dedicated to the determination of the structural properties of solids at high pressure using angle-dispersive diffraction with diamond anvil cells. The beam from an in-vacuum U20 insertion-device is focused by a vertical and horizontal transfocator and monochromatised by a Si(111) Bragg monochromator. The working energy for high-pressure experiments is 30 keV with a flux of 1012 photons/s at 200 mA. The beam size on the sample is normally about 5x5 µm2 but can be made as small as 1x1 µm2 for megabar pressure experiments. The scattered radiation is collected by an Eiger2 9M CdTe pixel detector from DECTRIS. A laser spectrometer is available for pressure determination by the ruby fluorescence method. It is also possible to perform Raman scattering experiments simultaneously. The beamline is equipped with several membrane-type diamond anvil cells (0-100 GPa), a liquid He-cooled cryostat to perform high-pressure experiments at low temperatures (down to 10 K) and external resistive heating equipment for high temperatures up to 600 K. A Nd-YAG laser system is available externally for high-temperature annealing of samples inside the diamond anvil cell. |