- Home
- News
- Spotlight on Science
- When oil and water...
When oil and water do mix: the nanoscale structure of a surfactant-modified hydrophobic interface
29-04-2011
Scientists have succeeded in measuring the molecular-scale structure and thermodynamics of deeply buried oil/water interfaces decorated by ionic surfactants. They found evidence of the interfacial layer becoming more dense, more ordered, than the oil layer as temperatures are reduced.
Share
Hydrophobicity plays a dominant role in fields ranging from the structure of living matter, like cell membrane stabilisation and protein folding, to microemulsion-mediated nano-particle formation [1]. Surfactants can be used to reduce the hydrophobic barrier between oil and water, as demonstrated by the millennia-long use of detergent-containing water to solubilise and remove oils from fabrics, crockery and the human body. Nevertheless, very challenging experiments are required that prevented, until recently, a molecular-resolution determination of the structure of the prime surfactant-modified hydrophobic interface between water and oil. Thus, a key ingredient in the fundamental understanding of the relation between surfactants and the hydrophobic interaction was still missing.
In a study published in the Proceedings of the National Academy of Sciences, a multi-continental collaboration of scientists from Israel, USA and the ESRF has succeeded in measuring for the first time the molecular-scale structure and thermodynamics of deeply buried oil/water interfaces decorated by ionic surfactants. The study employed X-ray reflectivity at the high energy microfocus ID15A beamline and surface tensiometry.
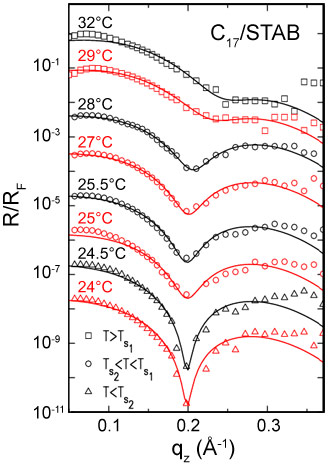
The interface between bulk oil (alkane) and pure water is simple, showing a monotonic transition from the density of oil to that of water over a distance of a few angstroms, determined by the capillary waves decorating all liquid interfaces [2]. When the pure water phase is replaced by a very dilute, sub-millimolar concentration, water solution of an alkyl ammonium bromide cationic surfactant, an intriguingly complex interfacial structure is found. At high temperatures, a liquid-like layer of density slightly higher than that of the bulk alkane is found to intrude between the alkane and solution bulk phases. This is manifested by the appearance of a shallow dip in the X-ray reflectivity (Figure 1). The layer consists of a mixture of alkane molecules and surfactant tails, and has a thickness smaller than the length of a single extended alkane molecule. Upon cooling this layer undergoes an abrupt freezing transition to a solid monolayer of densely packed, fully extended, interface-normal alkane molecules. This transition is observed in Figure 1, as a sharpening of the dip at ~28ºC and its shifting to a lower scattering vector q. Moreover, about 3ºC below this transition, a second transition is observed, manifested in an abrupt sharpening of the dip, but no shift in its position. This transition is very likely to originate from a rotator to a fully crystalline in-plane ordered phase. This interpretation is supported by the magnitude of the entropy loss in the transition, measured from the slope change occurring at the transition in the linear temperature dependence of the surface tension.
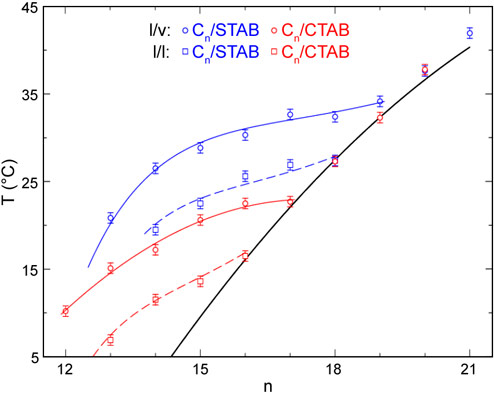
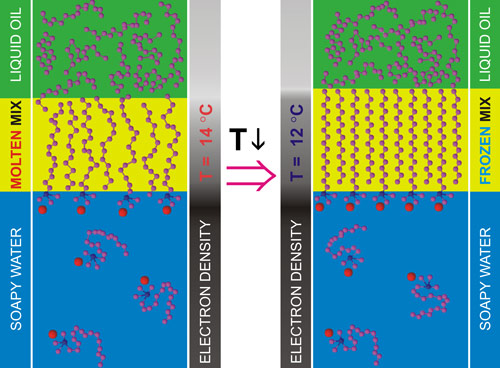
The temperature-dependent surface tension measurements, carried out for a range of alkane lengths (n) for two different-length surfactants (CTAB and STAB), determined the phase diagram of the interface. This phase diagram, shown in Figure 2, exhibits an increasing temperature range of existence for the interface-frozen monolayer with decreasing alkane length, reaching tens of degrees at low n. The transition temperature boundaries Ts(n) are similar to, but downshifted from, those observed for alkane monolayers spread on the liquid/vapour interface of the same solutions for both surfactants [3]. The study also offers a thermodynamic model for the transition, based on binary mixture theory and dominated by the interchange energy cost of replacing a surfactant tail with an alkane molecule. This model accounts well for the interfacial freezing transition at both the bulk alkane/solution and solution/vapour interfaces, as shown in Figure 2. The interfacial phase transition discovered in this work is summarised pictorially in Figure 3.
Extending this first molecular-resolution study to other surfactant-decorated hydrophobic interfaces should reveal how general is the behaviour found here, and allow a deeper insight into, and a deeper theoretical understanding of the microscopic origins of hydrophobicity in general.
Principal publication and authors
L. Tamam (a), D. Pontoni (b), Z. Sapir (a), S. Yefet (a), E. Sloutskin (a), B. Ocko (c), H. Reichert (b), and M. Deutsch (a), Modification of deeply buried hydrophobic interfaces by ionic surfactants, Proc. Nat. Acad. Sci. USA 108, 5522-5525 (2011).
(a) Physics Department and Institute of Nanotechnology, Bar-Ilan University, Ramat-Gan (Israel)
(b) ESRF
(c) Soft Condensed Matter & Materials Science Department, Brookhaven National Laboratory, Upton NY (USA)
References
[1] D. Chandler, Nature 437, 640 (2005).
[2] B.M. Ocko, X.Z. Wu, E.B. Sirota, S.K. Sinha and M. Deutsch, Phys. Rev. Lett. 72, 242 (1994).
[3] E. Sloutskin, Z. Sapir, L. Tamam, C.D. Bain, M. Willkinson, M.Deutsch, and B.M. Ocko, Phys. Rev. Lett. 99, 0136102 (2007).
Top image: At low temperatures, surfactant and oil molecules form a frozen, solid, 2-dimensional interfacial monolayer.