- Home
- News
- Spotlight on Science
- Structural and spectroscopic...
Structural and spectroscopic observation of an enzyme at work
16-09-2011
Observing enzymes at work is a difficult task. Yet, scientists at the University of Pavia in Italy, in collaboration with colleagues from the ESRF, the IBS and the University of Groningen in The Netherlands have succeeded in generating and characterising several biologically relevant intermediate states of a Baeyer-Villiger monooxygenase, a promising target for biocatalytic applications in synthetic and pharmaceutical chemistry. Their studies, combining X-ray crystallography, single-crystal microspectrophotometry, and X-ray induced photoreduction, have been published in the Journal of Biological Chemistry.
Share
At the end of the nineteenth century, Baeyer and Villiger discovered that cyclic ketones react with oxidants such as peroxymonosulphuric acid to yield lactones. Baeyer-Villiger reactions are of enormous value in synthetic organic chemistry and the number of their applications is countless. Several microorganisms produce enzymes able to catalyse Baeyer-Villiger reactions. Baeyer-Villiger monooxygenases are FAD-dependent proteins that use NADPH and molecular oxygen to insert an oxygen atom into their substrate.
We have identified a microbial Baeyer-Villiger enzyme, phenylacetone monooxygenase, which offers several unique and attractive features: it is thermostable and tolerant towards organic solvents, and catalyses enantioselective Baeyer-Villiger oxidations and sulfoxidations. Researchers from the University of Pavia in Italy, the ESRF, the Institut de Biologie Structurale in Grenoble, and the University of Groningen in The Netherlands were able to generate several intermediate states of the coloured enzyme phenylacetone monooxygenase and to characterise them by X-ray crystallography and single-crystal microspectrophotometry, both at the synchrotron beamline (online) and at the dedicated Cryobench laboratory (offline). The experimental strategy took advantage of otherwise detrimental X-ray induced changes to biomolecules to trigger reduction of the X-ray sensitive flavin moiety of phenylacetone monooxygenase (Figure 1).
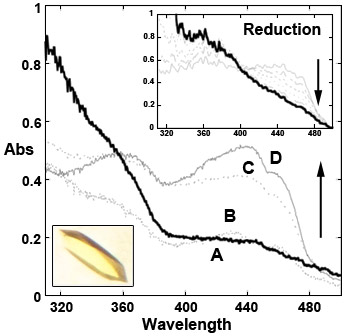 |
Figure 1. Microspectrophotometry of phenylacetone monooxygenase crystals (inset) measured at 100 K. X-ray exposure of the crystals leads to their reduction (spectrum A, bold line and inset). Reduced crystals can then be re-oxidised by soaking at room-temperature in aerated solutions as shown by spectra B, C, D that were collected on crystals cryo-cooled 2, 4, and 6 minutes after the beginning of re-oxidation. |
The intermediate state was captured and its structure characterised by collecting X-ray crystallographic data at a very low temperature of -170°C. These studies highlight the fascinating ability of Baeyer-Villiger monooxygenases to catalyse a complex multi-step reaction that originates from the concerted action of two cofactors (NADPH and FAD) and the protein active site to subsequently promote flavin reduction, oxygen activation, tetrahedral intermediate formation (Figure 2), and product synthesis and release. The emerging picture is that these enzymes are mainly oxygen-activating and “Criegee-intermediate stabilising” catalysts that act on any chemically suitable substrate that can diffuse into the active site, emphasising their potential value as toolboxes for biocatalytic applications.
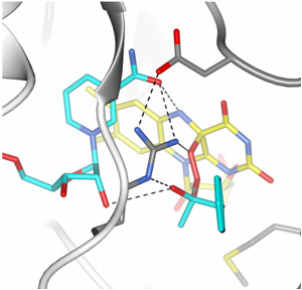 |
Figure 2. Model of the crucial oxygenating “Criegee intermediate” formed within the active site of phenylacetone monooxygenase. |
Principal publication and authors
R. Orru (a), H.M. Dudek (b), C. Martinoli (a), D.E. Torres Pazmino (b), A. Royant (c,d), M. Weik (c,d), M.W. Fraaije (b) and A. Mattevi (a), Snapshots of Enzymatic Baeyer-Villiger Catalysis: Oxygen activation and intermediate stabilization, J. Biol. Chem. 286, 29284 (2011).
(a) University of Pavia (Italy)
(b) University of Groningen (The Netherlands)
(c) Institut de Biologie Structurale, Grenoble (France)
(d) ESRF
Acknowledgement
Supported by the EU-FP7 project “Oxygreen”.
Movie summarising this research
|
|



