- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2000
- Soft Condensed Matter
- Lipid Membranes on Solid Surfaces
Lipid Membranes on Solid Surfaces
As the basic building blocks of biological membranes, the structure and physical properties of lipid bilayers are of great interest. Apart from the predominant role in the organisation of the biological cells, lipid bilayers can be expected to become a key component of novel biomolecular materials. In particular, solid-supported lipid bilayers may provide a way to biofunctionalise solid state and semiconductor surfaces, providing a compatible interface between the inorganic and biomolecular world [1]. Importantly, structural studies by X-ray scattering can be carried out at physiological conditions of hydration, temperature and ionic strength.
To investigate the thermal stability and fundamental fluctuation properties of solid supported lipid bilayers, we have carried out a series of specular and nonspecular X-ray reflectivity measurements using 20 keV synchrotron beams to probe phospholipid samples fully immersed in water, see Figure 26. Data was collected mainly on dimyristoyl-sn-glycero-phosphocholine (DMPC) and oleoyl-palmitoyl-sn-glycero-phosphocholine (POPC). Reproducibly, we could observe a transition from a discontinuous unbinding transition from a substrate bound, multilamellar state to a state of freely dispersed bilayers in water [2]. In the unbound phase, a single membrane remains on the substrate.
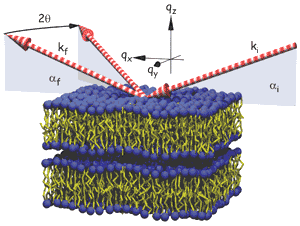 |
Fig. 26: Fluctuating multilamellar membranes, schematically shown with incident, reflected and diffracted beams.
|
The experimental observations are in line with theoretical predictions, according to which the entropic forces generated by the fluctuating membranes may lead to an unbinding of the membranes from each other and/or the substrate. However, the temperature of the transition was found to depend not only on sample thickness, but also on sample history (temporal and thermal), pointing to strong kinetic effects.
To probe the thermal fluctuation spectrum below and at the transition and to gain an understanding of the tremendous amount of nonspecular (diffuse) scattering in these systems, a high-resolution mapping of the scattering distribution both in and out of the plane of incidence was carried out at beamline ID1, using both a fast scintillation and a multiwire area detector. A representative data set for DMPC in the fluid La-phase is shown in Figure 27. Apart from pronounced conformal bilayer fluctuations giving rise to the diffuse Bragg sheet (BS) scattering, a weak contribution of static (topological) defects in the lamellar stack appears as a Debye-Scherrer ring (D), but anneals at higher temperatures.
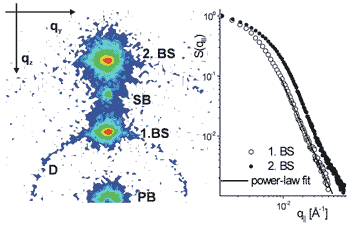 |
Fig. 27: (left) Typical scattering distributions for DMPC in the fluid L
|
A line scan parallel to the first and second diffuse Bragg sheet is shown in Figure 27 (right). From this data as well as complementary neutron scattering data, the membrane fluctuation spectrum g(r) can be determined, and thereby also elasticity parameters such as bilayer bending rigidity K and compressional modulus B as a function of T.
The results may be put in perspective to the relevant microscopic interactions and also be compared to current theories of membrane fluctuations and defects [3]. In the future, similar experiments may be applied to more complex lipid-membrane systems, including lipid/peptide, lipid/protein or lipid/DNA assemblies. The technique can reveal lateral structures (height and density correlation functions) on length scales between a few Å up to several mm at in situ conditions.
References
[1] T. Salditt, Curr. Op. Coll. & Interf. Sc. 5, 19 (2000).
[2] M. Vogel, C. Münster, W. Fenzl and T. Salditt, Phys. Rev. Lett. 84, 390 (2000).
[3] T. Salditt, C. Muenster, J. Lu, M. Vogel and W. Fenzl, Phys. Rev. E 60, 7285 (1999).
Principal Publication and Authors
M. Vogel (a,b), C. Münster (a), W. Fenzl (b), D. Thiaudere (c) and T. Salditt (a,d), Physica B 283, 32 (2000).
(a) Universität München (Germany)
(b) Max-Planck-Institut für Kolloid- und Grenzflächenforschung Golm (Germany)
(c) ESRF
(d) now at: Universität des Saarlandes (Germany)



