- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2004
- High Resolution and Resonance Scattering
- Collective Modes and Localised Vibrations in Methane Hydrate
Collective Modes and Localised Vibrations in Methane Hydrate
Methane hydrate [1] is an inclusion compound, in which methane molecules are trapped in an ice-like host lattice of water molecules. Three different crystallographic structures of methane hydrate are known: a low pressure phase MH sI, consisting of six large and two small cages in the unit cell, and two high pressure phases MH II and MH III. Methane hydrate structure I is of special interest as it forms at water-gas pressure conditions found in the world's oceans. Since large natural deposits have been discovered on the ocean floors, methane hydrate is considered as a potential future energy source. These hydrate deposits are detected by the difference in the sound velocities of the hydrate and gas baring sediments. Furthermore, the hydrate cemented sediments display mechanical properties, which are strongly dependent on the stability and the physical properties of methane hydrate. The unusually low and glass-like thermal conductivity observed in gas hydrates is therefore of special importance. This anomalous behaviour is thought to be attributed to a resonant scattering of the heat carrying acoustic lattice phonons by the localised guest vibrations, which should be observable in the phonon dispersion relation.
High energy resolution inelastic X-ray scattering (IXS) is therefore used to determine the collective dynamics of both the methane guest molecules and the host lattice. The phonon dispersion relation of methane hydrate was recorded at the beamline ID28. Figure 12 shows a selection of the recorded IXS spectra, which display a well defined dispersive mode and a second non-dispersive peak from 3 nm-1 on. The dispersive excitation is identified with the longitudinal acoustic host-lattice phonon branch. From the slope of the dispersion an orientationally-averaged sound velocity v = 3950 ± 50 m/s can be deduced. The peak at 5 meV is attributed to methane molecule vibrations inside the large cage. These vibrations become visible in the spectra after the intersection with the longitudinal acoustic mode at Q ~ 2.5 nm-1, which points toward a coupling between the localised guest vibrations and the dispersive acoustic host lattice modes.
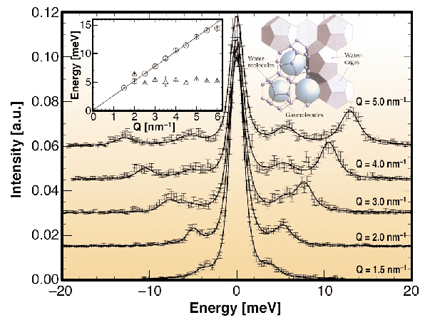 |
Fig. 12: Inelastic X-ray spectra of MH sI. The insets show the respective dispersion relation and a schematic of MH sI (provided by GEOMAR). |
This interpretation is validated by the theoretical calculated dispersion relation and IXS intensities using lattice dynamical simulations. The resulting scattering function, which was orientationally averaged in order to simulate the powder sample, is shown in Figure 13. It shows two distinct excitations, a dispersive mode, which is the longitudinal acoustic lattice mode, and an optic mode corresponding to the localised methane molecule vibrations. The calculated longitudinal sound velocity is 3800 m/s in agreement with the experimental value. The calculated energy position of the guest mode is lower than the observed one, which points towards an underestimation of the methane-water interaction. Nevertheless, the combination of experimental and theoretical results shows that before the cross-over of the guest and host modes, the guest molecules are independent Einstein oscillators, leading to a negligible intensity in the IXS spectra. It is at the cross-over that the guest mode acquires a collective behaviour as it is modulated by the host lattice mode. This coupling of the guest and host vibrations leads to a transfer of scattered intensity from the acoustic lattice mode to the optic guest mode and thus to a damping of the intensity of the acoustic lattice phonons, which are closely related to the thermal conductivity.
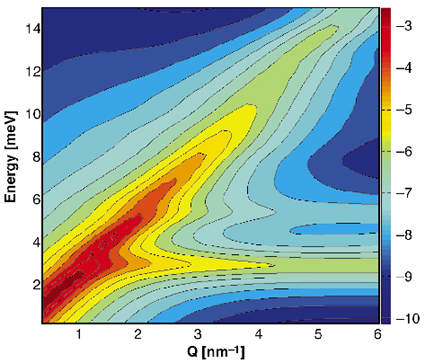 |
Fig. 13: Theoretical inelastic X-ray scattering function of methane hydrate. The longitudinal acoustic lattice mode and the optic guest mode can be observed. |
This coupling is not observed for the high pressure structures of methane hydrate, as the guest molecules cannot be considered as independent oscillators anymore. Instead, especially for the "filled ice" MH-III, strong changes in the elastic properties of the ice host lattice are observed. These changes can be connected with the growing hydrophobic interaction between the methane and water molecules.
In conclusion, it was possible to gain insight into the origins of the anomalous thermal conductivity of methane hydrate. The results can be used as a starting point for a modelling of the thermodynamic properties of natural occurring hydrate deposits. The pressure dependent studies could also show that the hydrophobic interaction between the guest and host molecules is of fundamental importance for the elastic properties of methane hydrate.
References
[1] E.D. Sloan, Jr., Clathrate Hydrates of Natural Gases (Dekker, New York, 1998).
Principal Publication and Authors
J. Baumert (a), C. Gutt (b), V.P. Shpakov (c), J.S. Tse (d), M. Krisch (e), M. Müller (a), H. Requardt (e), D.D. Klug (d), S. Janssen (f), and W. Press (g), Phys. Rev. B 68, 174301 (2003).
(a) Experimentelle und angewandte Physik, Universität Kiel (Germany)
(b) Experimentelle Physik I, Universität Dortmund (Germany)
(c) Chemistry Department, University of Western Ontario (Canada)
(d) National Research Council of Canada, Ottawa (Canada)
(e) ESRF
(f) Paul Scherrer Institute, Villigen (Switzerland)
(g) ILL



