- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2007
- Materials Science
- Studying a catalyst while it grows
Studying a catalyst while it grows
Zeolites are a family of tectosilicates that contain porous channels up to about 2 nm in size. These channels confer on zeolites properties such as molecular sieving and shape-selective catalysis, which have been widely exploited by the chemical industry. Controlling the outcome of the chemical synthesis, through an understanding of the formation and growth mechanisms, has therefore become one of the primary goals of the zeolite synthesis scientific community over the past 50 years. The rational design of such materials aims to improve their catalytic performance, amongst other things. A very powerful but relatively unexplored way of probing the processes involved in zeolite synthesis is to perform in situ studies during crystallisation. This is particularly powerful when complementary in situ measurements are performed using either a variety of techniques or when multiple techniques are combined into one setup.
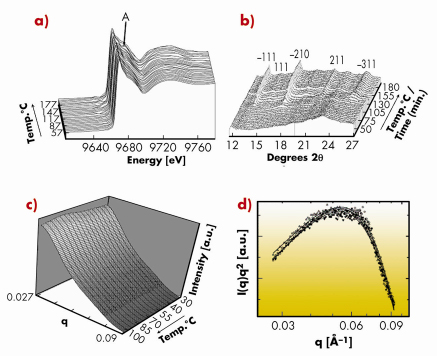
Recently we have developed a setup that combines small-angle X-ray scattering (SAXS), wide-angle X-ray scattering (WAXS) and X-ray absorption spectroscopy (XAS) and have used it to study the crystallisation processes leading to the formation of a zinc aluminophosphate zeotype (ZnAlPO-34) at BM26, the DUBBLE beamline. This combination of techniques allowed us to follow the changes that occur with time at the molecular, nanoscopic and crystalline level, with a time resolution in the order of a few minutes and represents, to the best of our knowledge, the first data of its kind acquired in such a manner, Figure 31.
 |
|
Fig. 31: (a) In situ Zn K-edge XANES with arrow marking the multiple scattering feature at ~ 9.685 keV; (b) WAXS with reflections indexed to ZnAlPO-34; (c) raw SAXS data; (d) I(q)*q2-log(q) plots (Kratky type plot) from the SAXS data collected during crystallisation. |
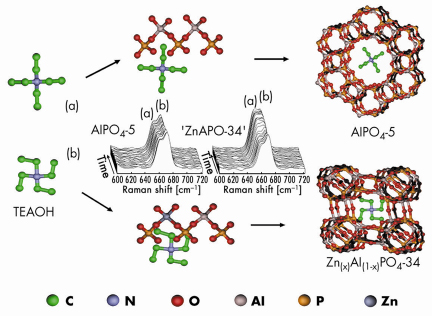
Growth of the zeolite occurred via a two-step aggregation/crystallisation process. The similarity of the average aggregate size measured by SAXS (ca. 12.3 nm) and WAXS (also ca. 12 nm) suggested that size retention occurs during crystallisation, implying that either critically-sized aggregates form in the gel structure or else a sort of amorphous-to-crystalline transition leads to the formation of a ZnAlPO-34 material. The growth of this crystalline phase began in preference to the AlPO-5 (AFI) structure despite the synthesis gel and conditions being typical to those used previously for AFI formation. Further measurements using in situ Raman spectroscopy demonstrated that an important interaction between the Zn2+ ions present and the tetraethylammonium hydroxide (TEAOH) template occurred. Changes in the Raman spectra were accompanied by the formation of a –34 type framework over the commonly occurring –5 type and hinted that such an interaction is critical for the formation of the –34 type structure, Figure 32. Zn2+ ions therefore appear to act as both nucleating agents and exert some structure-directing effect for the formation of the ZnAlPO-34 (CHA) phase. Clearly this setup is capable of providing a lot of congruent, simultaneous information in one experiment making it a new and powerful tool for studying crystallisation processes of molecular sieves. Futhermore, its application need not be restricted to this field alone since it could also provide information on the assembly processes of other crystalline and nano-structured materials.
 |
|
Fig. 32: Schematic diagram detailing the formation of microporous aluminophosphate types –5 and –34. The stacked Raman data in the middle suggest that TEAOH forms an intermediate complex with the aluminophosphate units and, depending on the nature of the TEAOH conformer (a or b), leads to either AlPO4-5 or ZnAPO-34 formation. |
References
[1] A.M. Beale, A.M.J. van der Eerden, S.D.M. Jacques, O. Leynaud, M.G. O’Brien, F. Meneau, S. Nikitenko, W. Bras, B.M. Weckhuysen, J. Am. Chem. Soc. 128, 12386 (2006).
[2] M.G. O’Brien, A.M. Beale, C.R.A. Catlow, B.M. Weckhuysen, J. Am. Chem. Soc. 128, 11744 (2006).
Principal publication and authors
A.M. Beale (a), A.M.J. van der Eerden (a), S.D.M. Jacques (b), O. Leynaud (b), M.G. O’Brien (b), F. Meneau (c), S. Nikitenko (c), W. Bras (c) and B.M. Weckhuysen (a)
(a) Inorganic Chemistry and Catalysis, University of Utrecht (The Netherlands)
(b) UCL London (UK)
(c) DUBBLE Beamline, ESRF



