- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2011
- X-ray imaging
- The darkening of chrome yellow pigments in paintings by Vincent van Gogh
The darkening of chrome yellow pigments in paintings by Vincent van Gogh
Lead chromate based compounds, commonly known as chrome yellow, belong to a class of pigments based on either lead chromate (PbCrO4, yellow and found in nature as the rare mineral crocoite), lead chromate–oxide [(1-x) PbCrO4·x PbO, yellow-orange and found in nature as the mineral phoenicochroite with structure Pb2O(CrO4)], in which the lead oxide conveys a reddish shade, or lead chromate sulfate [(1-x) PbCrO4·x PbSO4], in which the sulfate compounds are employed to obtain paler shades of yellow. Chrome yellow was intensively used both by artists of the end of 19th century (such as V. van Gogh, G. Seurat, J.M.W. Turner, J. Constable, P. Cézanne, C. Pissarro and J. Ensor) and for industrial purposes (painting of vehicles and air planes, road paint and signs). The darkening of this pigment under sunlight was known since its invention in the first half of the nineteenth century. This phenomenon is currently observable on many oil paintings by the Masters mentioned above and among them, the most famous are the different versions of Sunflowers by V. van Gogh (1853-1890). Although the scientific community suspected that this degradation involves a reduction reaction of the original Cr(VI) to Cr(III), the exact alteration mechanism and the resulting Cr(III)-alteration products have never been elucidated before.
Investigations at beamline ID21 by means of microscopic X-ray absorption near-edge (SR µ-XANES) and X-ray fluorescence spectrometry (SR µ-XRF) around the Cr K-edge on artificially aged model samples of commercial lead chromate and two darkened paint cross-sections taken from paintings by V. van Gogh (Bank of the Seine, F293, 1887 and View of Arles with Irises, F409, 1888 - Van Gogh Museum, Amsterdam, NL) allowed it to be demonstrated for the first time that the alteration of chrome yellow is caused by the reduction of PbCrO4 to Cr2O3·2H2O (viridian green).
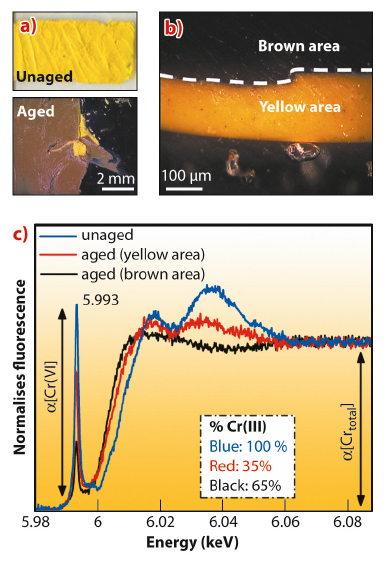
Among the UVA-visible aged model samples, only the one from an oil paint tube belonging to the Flemish Fauvist Rik Wouters (1882-1913) and containing a high amount of PbSO4 proved to be particularly prone to discoloration (Figure 141a-b). A strong spatial correlation between the presence of Cr(III) compounds at the surface of the chrome yellow paint layers and the darkening was found; this was also clearly demonstrated on the Cr K-edge XANES spectra by a decrease of the intensity of the pre-edge peak at 5.993 keV and a shift of the absorption edge toward lower energies (Figure 141c).
 |
|
Fig. 141: a) Image of paint model sample taken from a historic 19th century paint tube before and after the ageing process. b) Sample of the same aged material shown in panel (a), embedded in cross-section. c) Cr K-edge XANES spectra acquired at ID21 on the model sample before and after ageing process and estimation of relative Cr(III) percentage abundances. |
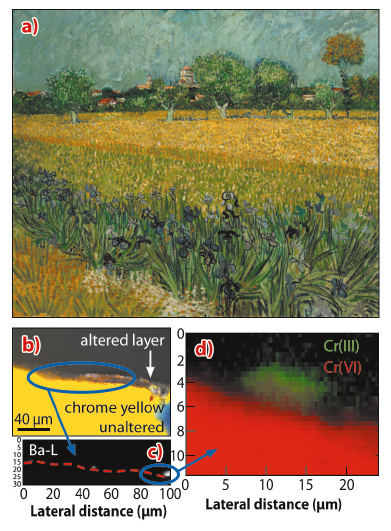
Analogous with this historic aged model sample, Figure 142 illustrates that X-ray analysis performed on the two Van Gogh painting micro-samples revealed at the altered exposed surface the presence of Cr(III) species in areas rich in Ba and S and/or aluminum-silicate compounds, while the surrounding yellow paint contained exclusively Cr(VI).
 |
|
Fig. 142: a) Photograph of View of Arles with Irises by Vincent van Gogh (1888, F 409, Van Gogh Museum, Amsterdam). b) Visible light microscopy image and c) grey scale image of Ba distribution (map size: 30 x 100 µm2; pixel size: 0.4 x 1 µm2; dwell time: 0.3 s). d) RG Cr-chemical state maps (size: 11.6 x 24 µm2; pixel size: 0.4 x 0.4 µm2; dwell time: 0.3 s). The mapped area is shown in (c) by a blue oval. |
Based on these results, we could arrive at the tentative assumption that SO42- anions are involved in some manner in the alteration mechanism of PbCrO4, without however being clear about the exact role they play. In this respect, a hypothesis was formulated: in the presence of abundant sulfate ions, a minor amount of sulfide ions can originate locally (chemical or microbial) that might act as reducing agent for the chromate ions via the regeneration of sulfates in an acidic medium. To prove this aspect of the degradation mechanism, a systematic additional study is underway.
Principal publication and authors
L. Monico (a, b), G. Van der Snickt (b), K. Janssens (b), W. De Nolf (b), C. Miliani (c), J. Verbeeck (b), H. Tian (b), H. Tan (b), J. Dik (d), M. Radepont (b,e) and M. Cotte (e,f), Anal. Chem. 83, 1214-1223 (2011); L. Monico (a,b), G. Van der Snickt (b), K. Janssens (b), W. De Nolf (b), C. Miliani (c), J. Dik (d), M. Radepont (b,e), E. Hendriks (g), M. Geldof (h) and M. Cotte (e, f), Anal. Chem. 83, 1224-1231 (2011).
(a) University of Perugia (Italy)
(b) University of Antwerp (Belgium)
(c) Istituto CNR di Scienze e Tecnologie Molecolari (CNR-ISTM) (Italy)
(d) Delft University of Technology (The Netherlands)
(e) C2RMF (France)
(f) ESRF
(g) Van Gogh Museum (The Netherlands)
(h) The Netherlands Cultural Heritage Agency (RCE) (The Netherlands)



