- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2011
- Structure of materials
- Occlusion of copolymer micelles in calcite single crystals generates an artificial biomineral
Occlusion of copolymer micelles in calcite single crystals generates an artificial biomineral
The composite structure of biominerals endows them with mechanical properties far superior to their synthetic counterparts. This derives from the association of organic molecules with the mineral host, where the organics can be located not only between crystalline units, as occurs in a structure such as nacre, but also within single crystals, as occurs for example in sea urchin spines. Indeed, single crystal biominerals can occlude organic macromolecules of levels of a few wt%, which is surprising given that crystallisation is a traditional method for purifying solids.
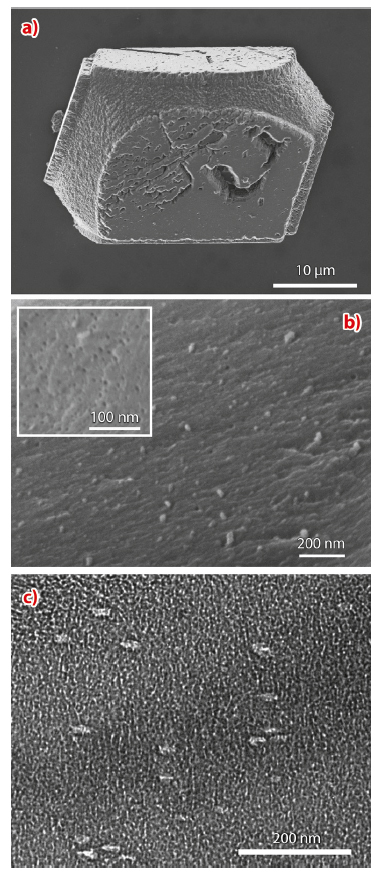
We have attempted the formation of an “artificial biomineral” by the encapsulation of organic additives within single crystals. Calcite crystals were precipitated in the presence of 20-30 nm anionic diblock copolymer micelles which exhibit a coronal layer of anionic carboxylated chains at pH values above ≈ 7. The crystals were principally 30-50 µm rhombohedral calcite (Figure 29a) and examination of cross-sections through fractured crystals revealed highly efficient occlusion of the polymer micelles at levels of a 20-30 vol.% (Figure 29b). IR spectroscopy revealed that the crystals possess a level of atomic disorder comparable with biogenic calcite.
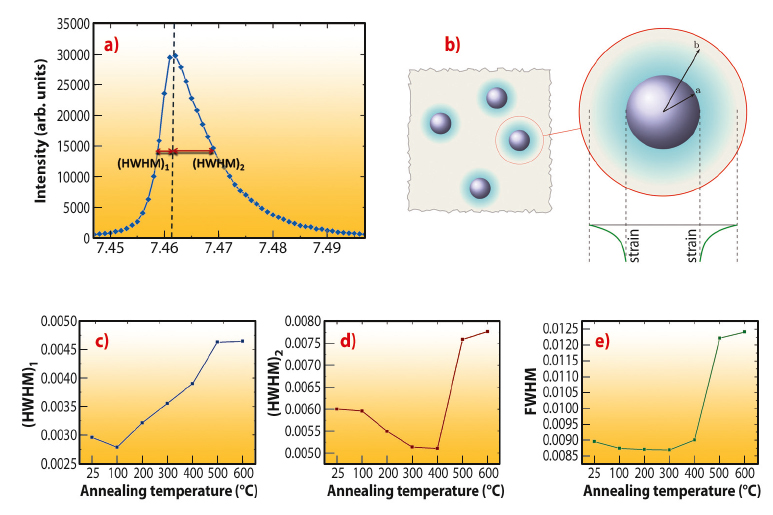
The influence of the particle inclusions on the calcite lattice was also investigated using synchrotron high-resolution powder diffraction (XRD) at beamline ID31 (Figure 30). In contrast to pure synthetic/geological calcite, which gives isotropically-shaped diffraction peaks, the peaks from the nanocomposite crystals were highly anisotropic and showed larger broadening towards higher 2θ (smaller d) values. This demonstrates the presence of a high compressive strain gradient in the calcite lattice, decreasing from a maximum at the calcite-micelle interface.
Concomitant heat treatment and XRD analyses of the samples were performed for temperatures between 100°C and 600°C. Two phenomena occurred on heating, namely a reduction in the peak anisotropy up to 300°C, followed by a general broadening of the peaks with further heat treatment. The decrease in peak anisotropy can be attributed to softening and then decomposition of the particles on heating, which reduces their effect on the crystal lattice. Peak broadening on annealing, in contrast, is unusual and “conventional” materials typically exhibit the opposite effect due to a reduction in the number of defects and an increase in grain sizes on heating. Interestingly, biogenic calcite and aragonite – which also occlude organic macromolecules – exhibit the same broadening effect. The observed broadening in our “artificial biomineral” can therefore be ascribed to an increase in the micro-strain fluctuations/free surface of calcite after the organics are burned away.
TEM studies were also performed to investigate how the single crystal lattice accommodates the micellar impurities. Imaging of thin sections through the nanocomposite crystals not only revealed the presence of the occluded particles, but also showed that they were anisotropic, with oval cross-sections (Figure 29c). Furthermore, the intra-crystalline particles were all co-aligned and were situated on {104} planes. The soft micellar particles therefore appear to undergo a change in morphology on binding to {104} faces during crystallisation, and subsequently become overgrown and occluded within the crystal.
Finally, the mechanical properties of the nanocomposite crystals were measured using nano-indentation methods. Comparison with pure synthetic calcite showed that the nanocomposite crystals were approximately 16% harder than their synthetic counterparts, and that cracks and steps appear at the indent impression in pure calcite which were not observed around the indent in the nanocomposite crystal.
Principal publication and authors
Y-Y. Kim (a), K. Ganesan (a), P. Yang (b), A.N. Kulak (a), S. Borukhin (c), S. Pechook (c), L. Ribeiro (d), R. Kröger (e), S.J. Eichhorn (d), S.P. Armes (b), B. Pokroy (c) and F.C. Meldrum (a), Nature Mater. 10, 890-896 (2011).
(a) School of Chemistry, University of Leeds (UK)
(b) Department of Chemistry, University of Sheffield (UK)
(c) Faculty of Materials Engineering and the Russell Berrie Nanotechnology Institute, Technion, Israel Institute of Technology, Haifa (Israel)
(d) School of Materials and the Northwest Composites Centre, Paper Science Building, Sackville Street, University of Manchester (UK)
(e) Department of Physics, University of York, Heslington (UK)