- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2014
- Structure of materials
- Positive thermal expansion of graphene on its substrate
Positive thermal expansion of graphene on its substrate
Graphene (a single sheet of graphite) is expected to exhibit unusual mechanical properties related to its atomic thickness. In a free-standing form, graphene is predicted to have a negative thermal expansion below a few 100 K, i.e. its lattice parameter should decrease as temperature increases (see ref. [1] and references therein). This behaviour is associated with the activation of out-of-plane vibrations when graphene is in a free-standing form. Whether such vibrations are still active when graphene rests on a substrate is unknown. To shed some light on this matter, we have studied graphene grown in situ on iridium. Graphene is known to have a weak interaction with this substrate.
We performed a series of in situ temperature-dependent high resolution surface X-ray diffraction experiments, from 10 to 1300 K, at beamlines BM32 and ID03, with high quality graphene. The graphene was prepared in situ on a (111)-terminated iridium crystal by chemical vapour deposition under ultra-high vacuum [2] to minimise the contribution from contaminants.
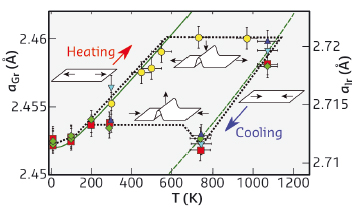
We found that graphene on its substrate exhibits no negative thermal expansion coefficient in the broad temperature range that we explored (Figure 127). The weak graphene-metal interaction indeed forces graphene’s lattice parameter to follow the temperature dependence of the metal lattice parameter.
 |
|
Fig. 127: Graphene lattice parameter (aGr, left axis) as a function of temperature. The different symbols refer to different samples prepared under the same conditions. The lattice parameter of the substrate (alr, right axis, same steps as left axis) as a function of temperature is also displayed as a solid green line. The dotted green is the same curve shifted to pass through the graphene data points. The cartoons show the appearance/disappearance of wrinkles in graphene, which merely compresses and expands in their absence. |
The lattice properties of the two materials are distinctive, and this shows up as graphene becomes unlocked from the metal lattice at a certain threshold, corresponding to a situation when the in-plane compressive (upon cooling down) elastic energy overcomes the sum of the binding and bending energies of graphene on iridium. Graphene forms linear delaminations: wrinkles several micrometres-long and a few nanometres-high that can be observed with a microscope.
 |
|
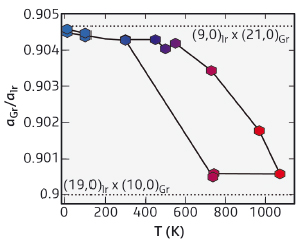
Fig. 128: Ratio of the graphene to iridium lattice parameters as a function of temperature. Dotted lines highlight low- and high-order lattice coincidences (commensurabilities). |
The transition between the two regimes – graphene locked and unlocked on its substrate – gives rise to a hysteretic change of graphene’s lattice parameter. Remarkably, after becoming unlocked from the substrate, graphene locks onto it again, adopting another, well-defined epitaxial relationship. At high temperature, this relationship approaches a coincidence of 21 carbon atoms with 19 iridium atoms, which is of high order, while at low temperature, the relationship is of lower order, with 10 carbon atoms matching 9 iridium atoms (Figure 128).
Principal publication and authors
F. Jean (a,b), T. Zou (c), N. Blanc (a,b), R. Felici (d), J. Coraux (a,b) and G. Renaud (c), Phys. Rev. B 88, 165406 (2013).
(a) Université Grenoble Alpes, Inst. NEEL, Grenoble (France)
(b) CNRS, Inst NEEL, Grenoble (France)
(c) CEA-UJF, INAC, SP2M, Grenoble (France)
(d) ESRF
References
[1] Y. Magnin, G. D. Förster, F. Rabilloud, F. Calvo, A. Zappelli and C. Bichara. J. Phys.: Condens. Matter 26, 185401 (2014).
[2] J. Coraux, A.T. N’Diaye, C. Busse and T. Michely. Nano Lett. 8, 565 (2008).



