- Home
- News
- Spotlight on Science
- Photosynthetic production...
Photosynthetic production of oxygen at elevated oxygen gas pressure
09-02-2009
Photosynthetic water oxidation at the manganese complex bound to photosystem II (PSII) from plants and bacteria is the source of the oxygen in the atmosphere. Oxidation state changes of the manganese atoms in the photosystem II protein during oxygen formation were studied by room-temperature time-resolved XAS experiments in the microsecond domain. The photosynthetic steps were found to proceed normally up to oxygen pressures of 16 bar.
The oxidation of two water molecules (H2O) by the abstraction of four electrons and four protons, whereby oxygen (O2) is a final product, is a particularly demanding chemical task. In Nature, only one enzyme, a reaction centre termed photosystem II (PSII), is capable of performing this reaction with high efficiency [1]. PSII can be found in plants, algae, and cyanobacteria in all kinds of terrestrial and aquatic ecosystems. Its activity, driven by the energy of absorbed light, has created the oxygen rich atmosphere that we enjoy today. Understanding the intricate mechanism of water oxidation is of key interest for future renewable fuel generation, where the electrons from water may be used to reduce protons by artificial photo-catalysts, thereby forming hydrogen (H2) in a reaction that is powered by the sun.
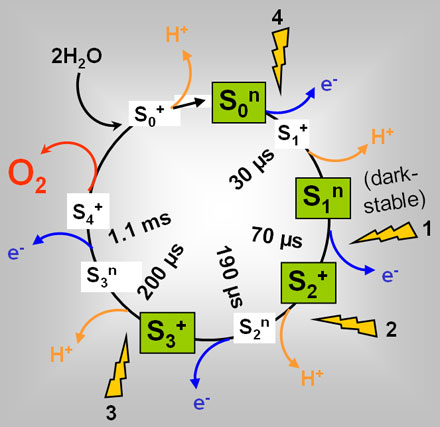
The water oxidising reaction is catalysed at a manganese-calcium (Mn4Ca) complex bound to the PSII protein (Figure 1). The successive absorption of three quanta of light by chlorophyll molecules in PSII leads to the stepwise removal of three electrons from the Mn complex until the so-called S3-state is reached. The absorption of the fourth photon and subsequent electron removal then leads to the oxidation of water by the previously oxidised Mn ions, to Mn re-reduction forming the S0-state, and to the concomitant production and release of an O2 molecule from PSII [2].
It has been hypothesised that the O2 formation step, like many other chemical reactions, may be reversible, meaning that the educt (the last reaction intermediate of Mn and bound water prior to O2 formation) and the product state (the re-reduced Mn complex in the S0 state plus an O2 molecule) are in equilibrium [3]. At ambient O2 partial pressure (pO2), the equilbrium may be expected to be largely biased towards the product state. However, under O2 enriched conditions, i.e. in future biotechnological reactors designed for maximal photosynthetic activity for fuel production or in certain natural environments (ancient or current), the increased pO2 may shift the equilibrium towards the educt, thereby leading to an unwanted lowering of the O2 production efficiency. Such an "O2 backpressure effect" may be the reason why the present atmospheric pO2 is only ~0.2 bar [3].
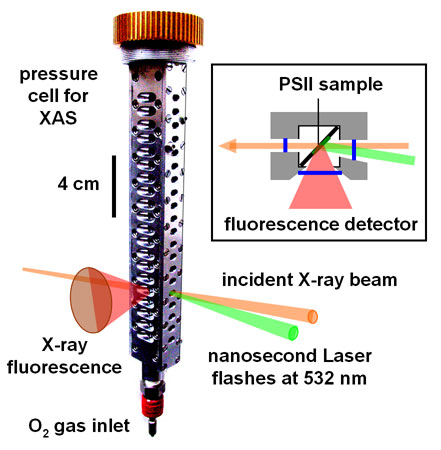
We monitored the rate and yield of manganese oxidation and reduction in the O2 formation step in isolated PSII protein from spinach in an X-ray absorption (XAS) experiment with time-resolution in the microseconds domain at beamline ID26 [4]. Previously developed techniques for time-resolved XAS were employed [5], e.g. a scintillation detector capable of recording kinetic data was used for the excited X-ray fluorescence. A pressure cell was constructed (Figure 2), which sustained O2 gas pressures up to ~20 bar. It allowed the Mn complex to be stepped through its reaction cycle by excitation with nanosecond laser flashes of PSII samples located in the pressurised cell and in the X-ray beam. XAS experiments were performed in the region of the Mn K-edge (~6550 eV), to ensure exclusive monitoring of the manganese redox reactions.
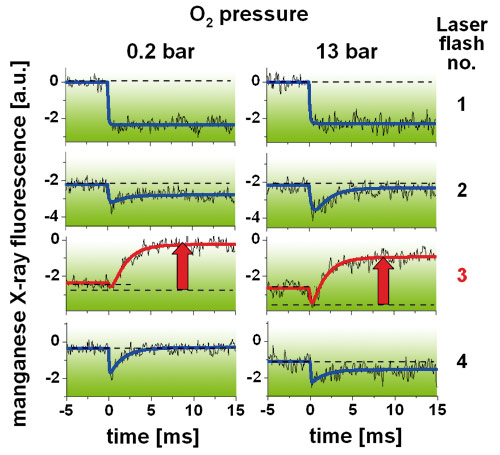
Measurements of XANES (X-ray absorption near edge structure) spectra and of the time course and amplitudes of X-ray fluorescence transients in each step of the water oxidation cycle (Figure 3) revealed that both the rates and yields of manganese oxidation, and of its reduction in the O2-forming step, were almost unchanged at O2 pressures up to 16 bar. Accordingly, there was no evidence that the efficiency of O2 formation was lowered by a thermodynamic O2 product inhibition. There were changes in the population of the S-states of the Mn complex in the dark, which lead to an altered synchronisation of the stepping through the manganese-oxidising reactions upon laser flash excitation of PSII. However, these effects presumably are attributable to (oxidative) reactions of manganese with reactive oxygen species (ROS) at elevated pO2, but not to a significant shift in the equilibrium of the O2 formation reaction itself.
Our results show that the O2 partial pressure in present ecosystems is unlikely to limit the photosynthetic activity as related to water oxidation and oxygen production by PSII. It remains to be elucidated, whether this O2 tolerance of the water oxidation reaction has always been a feature of the manganese complex, or whether it has evolved in the past four billion years since the origin of oxygenic photosynthesis, i.e. in parallel to the rise of the pO2 in the biosphere to its present level.
References
[1] B. Loll, J. Kern, W. Saenger, A. Zouni, J. Biesiadka, Nature 438, 1040 (2005).
[2] H. Dau, M. Haumann, Coord. Chem. Rev. 252, 273 (2008).
[3] W. Junge, J. Clausen, Science 312, 1470 (2006).
[4] M. Haumann, A. Grundmeier. I. Zaharieva, H. Dau, Proc. Natl. Acad. Sci. USA 105, 17384 (2008).
[5] M. Haumann, C. Müller, P. Liebisch, M. Barra, M. Grabolle, H. Dau, Science 310, 1019 (2005).
Principal publication and authors
M. Haumann, A. Grundmeier, I. Zaharieva, H. Dau, Photosynthetic water oxidation at elevated dioxygen partial pressure monitored by time-resolved X-ray absorption measurements, Proc. Natl. Acad. Sci. USA 105, 17384 (2008).
Physics Department, Freie Universität Berlin, Berlin (Germany)