- Home
- News
- General News
- Clear view of “Robo”...
Clear view of “Robo” neuronal receptor opens door for new cancer drugs
07-03-2019
Proper brain function depends on neurons forming accurate circuits in the brain, guided by special protein receptors that sense the environment around them. Scientists using ID29 have discovered the molecular mechanism that allows a key guidance receptor, “Robo”, to react to signals around it. Developing drugs to target the receptor could lead to promising treatments for cancer.
Share
During brain development, billions of neuron nerve cells must find accurate pathways in the brain in order to form trillions of neuronal circuits enabling us to enjoy cognitive, sensory and emotional wellbeing. To achieve this remarkable precision, migrating neurons use special protein receptors that sense the environment around them and guide the way so these neurons stay on the right path. In a new study published in Cell, researchers from Bar-Ilan University and Tel Aviv University in Israel, EMBL Grenoble in France and University of Exeter in the UK report on their discovery of the intricate molecular mechanism that allows a key guidance receptor, “Robo”, to react to signals in its environment.
One of the most important protein signaling systems that guide neurons consists of the cell surface receptor "Robo" and its external guidance cue, "Slit". “Slit and Robo can be identified in virtually all animals with a nervous system, from a 1 mm-long nematode all the way to humans,” explains researcher Yarden Opatowsky, associate professor and head of the Laboratory of Structural Biology at Bar-Ilan University and who led the research.
There has been a lot of progress towards understanding Slit-Robo function and the developmental responses that they trigger in the brain and the central nervous system. However, scientists still had an incomplete understanding of how Slit activates Robo and what keeps Robo inactive in the absence of Slit to prevent harmful activation, which has been implicated in kidney diseases and age-related macular degeneration. Furthermore, a deficit of either of these proteins results in defects in brain structure and function, so understanding how Robo and Slit function is of significant interest.
Opatowsky and colleagues had been collecting diffraction data from Robo crystals since 2009 but it was not until the team used the newly installed Pilatus 6M detector on ESRF beamline ID29 and at BESSY II in Germany that they were able to obtain diffraction data to under 4 Å resolution. Using molecular replacement, the team was able to solve the crystal structures of the intact Robo ectodomain (the extracellular portion). The structures indicated how two Robo receptors form active dimers and how their dimerisation interfaces are kept blocked in the absence of Slit.
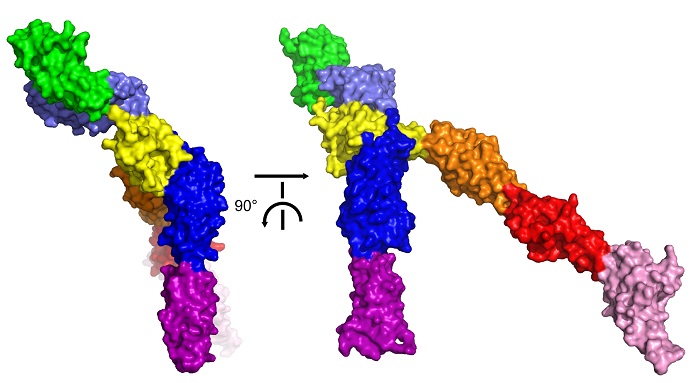
A surface representation of the crystal structure of the extracellular portion of human Robo2. The yellow region represents the domain where dimerisation takes place. Here, we see it blocked by the other domains, meaning dimerisation cannot take place and that Robo2 is inactivated. Credit: Y. Opatowsky.
“I cannot imagine a successful conclusion for this project without having an almost-immediate access to state-of-the-art facilities and, most importantly, to the brilliant and devoted beamline scientists at the ESRF and BESSY II,” Opatowksy says. However, his team's work did not end there. “It took us six years to get the desired structure, but it was just the first phase of our research,” he explains.
The key is in the worm
Following their crystallographic observations, Opatowsky’s team wanted to investigate their structural model in a living biological system. To test Robo’s function in vivo, the team used the C. elegans nematode – a tiny, multi-celled worm that is the only organism to have its complete neuronal ‘wiring diagram’ known. C. elegans has only one Robo and one Slit (compared to four Robo genes and three Slits in humans and mice), making it ideal to study.
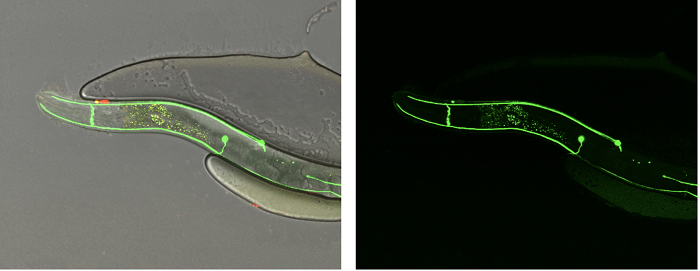
To test their structure-driven hypotheses, the researchers used the C. elegans nematode and followed the trajectories of neuronal extensions (in green) that indicate the activity or loss-of-function of Slit and Robo. Credit: Y. Opatowsky.
The evidence supported their structural model that Slit activates Robo through the release of an auto-inhibitory mechanism that keeps Robo inactive in the absence of Slit, and also showed that the same mechanism actually exists in other closely related receptors. “The evolutionary distance between humans and C. elegans is about 500 million years, and yet, all the experiments that we conducted point toward a similar Robo molecular mechanism,” says Opatowksy.
A drug target for cancer
The involvement of Slit and Robo receptors in cancer is of particular interest to researchers. In cancer, some receptors are ‘high-jacked’ to drive tumour formation. In personalised cancer therapy, drugs block these rogue receptors, depriving the cancer cells of vital signaling instructions and directing them toward destruction. Targeting Slit and Robo has long been considered a promising therapeutic approach for types of pancreatic, skin and breast cancer. However, and almost certainly due to an insufficient structural and mechanistic understanding of Robo activation and signaling, there are currently no Robo-directed drugs.
“Our discoveries provide, for the first time, the information necessary to design effective drugs targeting Robo receptors,” says Opatowsky. “Particularly, the crystal structures revealed molecular sites on the surface of Robo that, when targeted by designed drugs, will allow us to manipulate Robo activation and inhibition in patients, thus providing new possibilities for treatment in the war on cancer.”
Reference:
R. Barak et al., Structural Principles in Robo Activation and Auto-Inhibition, Cell, 7 March 2019.
Text: Anya Joly
Top image: Crystal structure of human Robo2 entangled with the C. elegans nematode axon guidance model that was used to test the structure-based hypotheses in vivo, on a background of a blooming field of mouse dorsal root ganglion growth cones poised for Slit-Robo stimulation and responses. Credit: Y. Opatowsky.



