- Home
- News
- General News
- How a protein mutated...
How a protein mutated in infertile women could become a non-hormonal contraceptive target
22-07-2019
An international team of scientists from the Karolinska Institutet in Sweden and Nagoya University has explained how mutations in egg coat protein ZP1 cause infertility in women. The study suggests that ZP1 could be a promising candidate for future non-hormonal contraceptive efforts.
Share
ZP1 is a glycoprotein involved in the fertilization of eggs by cross-linking egg coat filaments. Because studies in mice showed that lack of ZP1 reduces but does not abolish fertility, scientists believed that this molecule was also not crucial for fertility in humans. This new study, however, suggests that ZP1 may have a much more important role in human reproduction than previously thought. “The results were a big surprise because they suggested that mutations that truncate the human ZP1 protein cause female sterility by hindering its cross-linking function, rather than interfering with other egg coat proteins”, explains Luca Jovine, professor at the Karolinska Institutet and leader of the study.
The most important implication of the study, for which the team travelled to the ESRF to carry out X-ray crystallography on ZP1, is that ZP1-mediated cross-linking of egg coat filaments is crucial for forming a stable coat around human egg cells – a prerequisite for successful reproduction. The researchers identified the region of ZP1 that makes the cross-links and show that this is able to make flexible connections, which could be modulated by sugar residues attached to ZP1 as well as zinc ions secreted by the fertilized oocyte.
The structural biology experiments carried out at the ESRF’s ID23-1 beamline resulted in the structure of the cross-linking region of the protein in its fully glycosylated state, whose analysis explained yet another infertility-associated mutation of human ZP1.
Towards non-hormonal contraceptives?
Egg coat proteins that mediate fertilization have no other function in the body, as far as researchers know. “This makes them excellent targets for medicines and may lead to new contraceptives without problematic side effects”, explains Jovine.
ZP1, in particular, is present in even smaller amounts than other egg coat components. As its cross-linking function appears to be crucial for human fertility, and because the crosslinks are only made when the protein is secreted, future drugs interfering with ZP1 secretion or cross-linking could, in principle, prevent fertilization.
 |
|
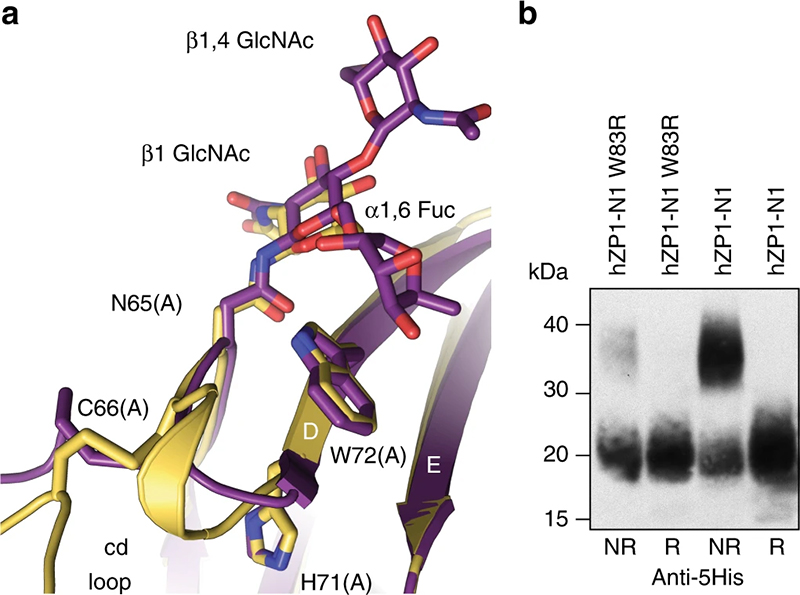
The mutation W83R of human ZP1 does not hinder its secretion but reduces its cross-linking (panel b), likely due to the fact that - as suggested by the structure of chicken ZP1 (panel a) - W83 (W72 in chicken ZP1) stacks between a sugar attached to ZP1 and the loop that makes the cross-link ("cd loop"). The part of the sugar chain that stacks against W83, which is a fucose residue, was only resolved in the structure of the fully glycosylated protein (violet) whose data came from ESRF ID23-1. |
“The ESRF has been a very important tool in my research throughout the years, as this paper also shows, and I am hoping that the new EBS will enable us to even strengthen this collaboration”, explains Jovine. “Because our crystals don’t always diffract well, having faster acquisition rates will definitely help by allowing us to screen a larger number of specimens. At the same time, in cases in which crystals are scarce, the fact that we will be able to screen at room temperature will avoid the risk of damaging a significant proportion of the samples while cryocooling them before the experiment”, he concludes.
Reference:
Nishimura, K. et al, NATURE COMMUNICATIONS | (2019) 10:3086. https://doi.org/10.1038/s41467-019-10931-5
Text by Montserrat Capellas Espuny
Top image: LEGO model of two egg coat filaments cross-linked by a pair of ZP1 proteins (yellow bridge). Credits: L. Jovine



