- Home
- News
- General News
- Learning from molluscs
Learning from molluscs
20-03-2012
Understanding how sea creatures engineer their superior armour inspires advanced materials, ranging from artificial bone to rock-hard semiconductors.
Share
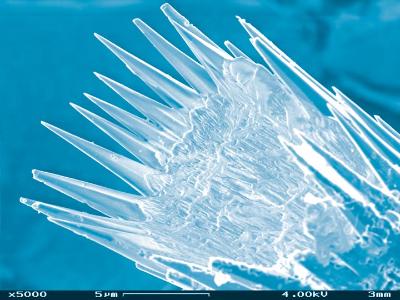
It’s hard not to marvel at the beauty of seashells, be it their mathematically intriguing spirals or iridescent mother-of-pearl coatings. But for some people, these surface properties are mere distractions. “I think mollusc shells are much more beautiful when you look at their structure under high magnification,” says materials scientist Boaz Pokroy of the Technion Israel Institute of Technology. “It’s amazing to see how relatively simple organisms have such a sophisticated way of producing functional materials from such basic ingredients, all at standard temperatures and pressures.”
Mollusc shells consist almost entirely of calcium carbonate, the same compound that makes up chalk. Whereas chalk is brittle enough to be broken by a nervous teacher at a blackboard, however, shells are tough enough to protect creatures from the grip of a hungry lobster or other dangers. Mother-of-pearl may look pretty, but the laminated organic–mineral structure responsible for its optical properties is perfect for diffusing cracks. Indeed, molluscs – a diverse class of organisms with tens of thousands of individual species – have survived for over half-a-billion years.
Now, thanks to synchrotrons, researchers are beginning to understand how nature engineers its biomineral armour. “We hope that our model systems, which are simpler than those in nature, will allow us to extract guiding principles that help us to understand biology,” says ESRF user Anna Schenk of the University of Leeds in the UK. “But we also want to use those principles to help us to design materials and minerals that are technically more relevant – it’s fascinating to find out the processes that govern these beautiful structures that are so hard to replicate in the laboratory.”
Biomimetics
The secret to biomineralisation lies in how a tiny amount of organic material, such as a protein, gets incorporated into the crystal structure of a mineral rather than simply acting as glue between crystallites. Experiments carried out at the ESRF during the past five years show that proteins are incorporated into calcium carbonate at the atomic level. Precisely how this happens, giving shells their impressive mechanical properties, is not fully understood. “Another open question is how proteins recognise the crystallographic surfaces of minerals in the first place,” explains Pokroy. “A protein is a large molecular soft-matter object that somehow can bind to calcium carbonate or a silicon structure – and moreover, it can bind to a specific crystallographic facet.”
Experiments at the ESRF’s ID31 beamline have gone some way to answer these fundamental questions, while ID22 has enabled non-destructive, high-resolution imaging of biogenic crystals and structures in 3D. Pokroy and co-workers, who include Emil Zolotoyabko of the Technion and Andy Fitch of the ESRF, have shown for instance that polymers introduce different lattice strains and thus distortions into the mineral crystal. In addition to providing geologists with a technique to differentiate between biological and geological material, such results could help identify what needs to be tuned in technological materials to achieve desired properties.
Calcium phosphate associated to hydrogels, for instance, can be used to make biocompatible bone-replacement materials. But there is also the possibility of transferring this additive-mediated mineral growth to metal oxides or semiconductors to make materials with better mechanical – or perhaps completely new – properties. The self-repairing nature of mollusc shells could even lead to “smart” engineering materials that repair themselves after failure.
“Research is explorative at the moment because we don’t understand very well yet what the crucial processes are,” says Schenk. “The impact of synchrotrons has been huge because you can study the sub-micron- and nano-structure, and learn about the crystallography of these samples in very small proportions. At ID31 the powder experiments are really well automated so you can measure many samples and look at the results online.”
Matthew Chalmers
This article appeared in ESRFnews, March 2012.
To register for a free subscription and to rapidly receive the current issue, please go to: ESRFNews Digital Edition Subscription.
Top image: Skin deep: only a serious microscope or synchrotron can reveal the true beauty of mollusc shells (Credit: Simon Ingate/istockphoto).