- Home
- News
- General News
- Keeping hormone...
Keeping hormone levels in check
25-05-2012
In plants, hormones are central to the regulation of growth, development and the defence response. The activity of many hormones is regulated by enzymes in the GH3 family. A team of researchers from the USA and France have investigated the structural basis for hormone modification by these critical regulatory enzymes and have provided the first atomic level structures of these novel proteins.
Share
Proteins from the GH3 family are able to selectively bind, activate, and modify plant hormones, tuning their in vivo activity. This process is tightly regulated, and critical for the plant, since plant hormones are active at nanomolar levels. GH3 proteins perform multiple reactions using a single active site. How the enzyme is able to selectively bind its substrates and catalyse different reactions was unknown. Now a team of researchers from Washington University (USA) and the ESRF and EMBL in Grenoble (France) have just published results in the journal Science that provides a structural insight into the catalytic activity of the GH3 proteins.
There are two steps in the chemical reaction catalysed by GH3 proteins. In the first step, the GH3 protein binds ATP (adenosine triphosphate, a source of energy within the cell) and the free acid form of plant hormones such as auxins, benzoates, and jasmonates and activates these molecules through the formation of an AMP (adenosine monophosphate) conjugate. In the second step, the protein selectively adds an amino acid to the activated hormone, forming a stable amino acid conjugate. Amino acid modification of plant hormones can lead to their inactivation, degradation, or increase their activity through binding to specific cellular receptors.
Crystal structures of two different GH3 proteins in complex with substrates and products and extensive mutagenesis studies have permitted the elucidation of the molecular basis for hormone selectivity, chemical activation, and amino acid conjugation in the GH3 protein family. These results demonstrate how a highly adaptable three-dimensional scaffold is used for the evolution of promiscuous activity across a enzyme family critical for modulating plant hormone action.
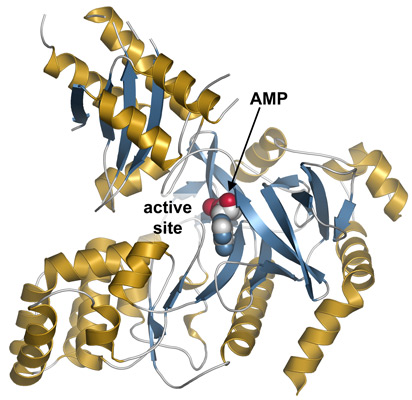 |
|
Structure of AtGH3.12. Ribbon diagram showing α-helices and β-strands coloured gold and blue, respectively and with AMP in the active site shown as a space-filling model. |
Chloe Zubieta, scientist in the ESRF Structural Biology Group, commented, “The success of the project relied on a multinational collaboration and took advantage of the facilities and expertise available at the ESRF, PSB (Partnership for Structural Biology) and EMBL, in particular the state of the art beamlines at the ESRF and the biophysical platform of the PSB.”
Protein expression, purification, crystallisation, and structure determination was performed in the Molecular Biology Laboratory at the ESRF. Using the high-throughput crystallisation platform of the PSB, thousands of different crystallisation conditions were screened. The structure of seleno-methionine derivatised GH3.12 was solved using data collected on the EMBL/ESRF MAD beamline BM14. This provided a molecular replacement model for all subsequent GH3 structures and the different substrate and product complexes. To collect the best quality data from sometimes poor crystals, helical data collection was used on the microfocus beamline ID23-2. This procedure was critical for minimising radiation damage and obtaining a high quality structure of the substrate complex of GH3.12 with AMPCPP (a non-hydrolysable ATP analogue).
By understanding the molecular basis for plant hormone modifications by enzymes such as the GH3 family, processes such as fruiting, seed development, and seasonal limitations in flowering for crop plants could be targeted to help address the ever-growing needs of an expanding human population and to achieve the goal of sustainable development and food production. This work not only illuminates an important family of enzymes which act directly on diverse plant hormones, but also will have future applications in plant breeding and domestication.
Reference
Structural Basis for Prereceptor Modulation of Plant Hormones by GH3 Proteins, C.S. Westfall, C. Zubieta, J. Herrmann, U. Kapp, M.H. Nanao, J.M. Jez, Science (24/05/2012) DOI: 10.1126/science.1221863.
Top image: Plant growth is kept in check by the GH3 family of proteins.



