- Home
- News
- General News
- X-rays create a...
X-rays create a window on glass formation
16-04-2012
First ever visualisation of how powder becomes molten glass could lead to greener manufacture at lower temperatures.
Share
Jointly published by the CNRS and the ESRF
Scientists have for the first time visualised the transformation of powder mixtures into molten glass. A better understanding of this process will make it possible to produce high quality glass at lower temperatures, leading to significant energy savings in industrial glass manufacturing. The results are published in the Journal of the American Ceramic Society.
The team of scientists was led by Emmanuelle Gouillart from the joint research unit between CNRS and Saint Gobain, a global glass manufacturer, and included scientists from the Universities of Toulouse and Grenoble, INRIA Saclay and the European Synchrotron Radiation Facility (ESRF) in Grenoble.
Glass is one of the oldest man-made materials, use of which spread during ancient Egyptian and Roman cultures. A non-crystalline amorphous material, it is produced by the fusion of crystalline powder mixtures heated to high temperatures. These ingredients are quartz sand (silica, SiO2), sodium and calcium carbonates (Na2CO3, CaCO3), and minor more specific additives.
In industrial foundries, the powder mixture is heated to about 1500°C and kept at this elevated temperature for many days to eliminate bubbles and unmolten grains. This consumes a lot of energy and one of the current industrial challenges is obtaining glass of good quality at lower temperatures. For example, the global glass industry’s energy consumption (86.5 TWh in 2005) compares with the entire electricity production of the Netherlands (108 TWh in 2008).
An individual grain of silica normally melts at very high temperatures (1700°C). Adding carbonates triggers chemical reactions that lower this temperature. However, the interplay between the geometry of the grains and the rate of chemical reactions during the early stages of the melting which starts already well below 1000°C, have remained a mystery to date.
The scientists set out to understand what exactly happens at the different stages of the transformation from powder to molten glass. For their experiment, they used mixtures of raw materials similar to that for making industrial window glass: two-thirds silica sand and one-third of sodium and calcium carbonates.
 |
Scientists setting up an experiment at beamline ID15 (Credit: ESRF/Molyneux Associates). |
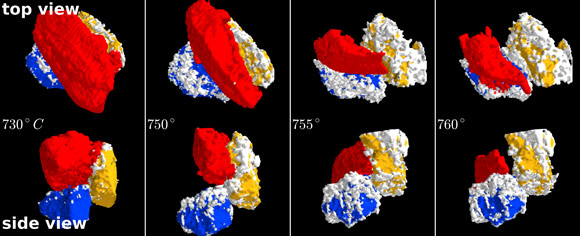
To make visible chemical reactions between individual grains, the scientists used X-ray microtomography, a technique allowing visualising in real time changes in shape and positions of all grains in a given volume. These changes are probed by a fine, intense beam of X-rays sent through the sample. Like a three-dimensional “frame by frame” sequence - tiny variations of the transmitted X-ray intensity are recorded when sand and carbonate grains start to react chemically, changing their shapes and transforming themselves into molten glass. “At the ESRF, we can take a microtomography image with a spatial resolution of 1.6 micrometres every few seconds. Observing fast changes with a high spatial resolution deep inside an oven held at close to 1000°C is impossible without X-rays”, says Marco Di Michiel from the ESRF.
The sequences of microtomography images confirmed the importance of good contact between grains of different substances, as it is these contacts which determine whether or not the mixture turns into liquid glass. For example, a calcium carbonate grain can either incorporate itself into the highly reactive amorphous liquid or remain a crystalline defect, depending on the presence or absence of such contacts. The researchers were surprised by the high reactivity of sodium carbonate when still solid: these grains move just before the melting begins which increases the number of contacts with other grains and facilitates the reactions.
By merging hundreds of X-ray tomography images, the scientists produced a video sequence visualising how different grains in the mixture move and fuse, one after the other, into molten glass as the temperature rose from 750°C to 930°C. “I have been working on these processes for many years, and it was absolutely fascinating to see like in a movie what happens at the onset of the powder/glass transition”, says Emmanuelle Gouillart.
The scientists now wish to vary the sizes of the grains and the way in which they ramp up the temperature. In the long term, these fundamental studies will tell us how to reduce the number of defects produced at the start of the glass formation process, and help to find faster and less energy consuming manufacturing processes. “We also wish to make X-ray imaging methods and data analyses a routine visualisation tool for reactive granular mixtures. These are not only used in the manufacture of glass but also of other materials, and I see a huge industrial potential for optimising these processes”, concludes Emmanuelle Gouillart.
Video of the transformation of the sand grains into glass (© E.Gouillart)
- View via YouTube: http://www.youtube.com/watch?v=pPyG_fEee_o
- Download in .avi format: http://www.esrf.fr/files/comm/tomo_glass_melting.avi
Reference
E. Gouillart et al., In-situ synchrotron microtomography reveals multiple reaction pathways during soda-lime glass synthesis, Journal of the American Ceramic Society, (2012).
Top image: Glass in the making - a grain of sodium carbonate (red) reacting with two grains of silica (blue and yellow).