- Home
- News
- Spotlight on Science
- Roughness as a motor...
Roughness as a motor for reaction oscillations
29-06-2010
Oscillatory chemical reactions are often called chemical ‘clocks’ due to their periodic nature. A well-known system that shows periodic oscillation of reaction rate is the catalytic oxidation of carbon monoxide over platinum and palladium surfaces. A team of researchers from Leiden University and the ESRF have pinpointed a new mechanism that makes this chemical clock ‘tick’.
Share
A catalyst is a substance that speeds up a chemical reaction without being consumed by the reaction. A well-know example is the catalyst in the exhaust of a car, which facilitates the oxidation of poisonous CO gas with oxygen to produce the less-harmful carbon dioxide. The rate of conversion of CO to CO2 can spontaneously oscillate in time. A complex interplay of the reactants, CO and O2, modifies the surface structure of the catalyst and thereby its catalytic activity. This causes the rate to oscillate for reactions at low pressures in ultrahigh vacuum systems, as has been beautifully demonstrated by Nobel laureate G. Ertl and co-workers [1]. However, at atmospheric pressures the structure of a catalyst surface is much harder to study because most analytical tools require low-pressure vacuum conditions.
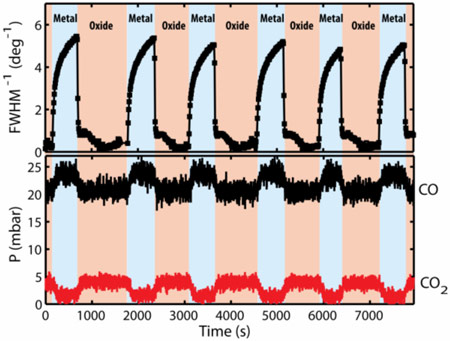
The Leiden University and ESRF team has developed a novel setup at beamline ID03 that allows them to study the surface structure of the single crystal Pd catalyst inside a flow reactor during the catalytic reaction by means of surface X-ray diffraction (SXRD) at atmospheric pressure [2]. With these SXRD experiments they show that during the rate oscillations the Pd catalyst spontaneously switches in time between a metallic surface, with a low CO2 production rate, and an oxidised Pd surface, which exhibits a much higher rate, as shown in Figure 1. These observations are in full agreement with earlier studies of the catalytic activity of ultrathin surface oxides [3,4].
Further SXRD experiments show that a smooth metal surface oxidises easier, i.e. at the less-oxidising conditions of a higher CO partial pressure, than a rough metal surface. The authors suggest that this is due to the favourable adsorption of CO at the steps of a rough metallic Pd surface, which thermodynamically stabilises the metal surface. Only when the level of roughness is sufficiently low does the surface becomes oxidised.
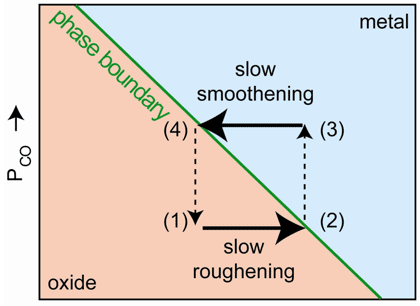
The newly discovered mechanism responsible for the oscillatory behaviour, depicted schematically in Figure 2, was found by studying the evolution of the roughness of the metallic and oxidised surface during the oscillations. In the oxide phase of an oscillation cycle, the thin oxide layer becomes gradually roughened by reaction of CO with the oxygen atoms from the oxide layer. This is shown by the broadening of the diffraction peaks. At a certain level of roughness the oxide layer becomes thermodynamically unstable and disappears, leaving a less reactive metallic surface. This metallic surface becomes smooth again by metal atom diffusion, as shown by the narrowing of the diffraction peak, see Figure 1. The smoothening continues until the roughness is low enough to make the oxide surface thermodynamically stable again, at which point the surface immediately forms a highly reactive oxide film. This completes the cycle: the process starts all over again from the beginning. Based on these observations the authors conclude that the evolution of the roughness is responsible for the periodic switching between the metallic and oxidised surface and is at the heart of the mechanism that makes this chemical clock tick.
 |
|
Figure 2. Metal-oxide stability diagram. Each cycle takes the surface through stages (1) smooth oxide, (2) rough oxide, (3) rough metal, and (4) smooth metal, after which the next cycle starts again at (1). The phase boundary is determined by the roughness and the CO partial pressure. |
References
[1] R. Imbihl, G. Ertl, Chem. Rev. 95, 697-733 (1995).
[2] R. van Rijn, M.D. Ackermann, O. Balmes, T. Dufrane, A. Geluk, H. Gonzalez, H. Isern, E. de Kuyper, L. Petit, V.A. Sole, D. Wermeille, R. Felici, and J.W.M. Frenken, Rev. Sci. Instrum. 81, 014101 (2010).
[3] H. Over, Y.D. Kim, A.P. Seitsonen, S. Wendt, E. Lundgren, M. Schmid, P. Varga, A. Morgante, G. Ertl, Science 287, 1474 (2000).
[4] M.D. Ackermann, T.M. Pedersen, B.L.M. Hendriksen, O. Robach, S.C. Bobaru, I. Popa, C. Quiros, H. Kim, B. Hammer, S. Ferrer, and J.W.M. Frenken, Phys. Rev. Lett. 95, 255505 (2005).
Principal publication and authors
B.L.M. Hendriksen (a), M.D. Ackermann (a,b), R. van Rijn (a,b), D. Stoltz (a), I. Popa (b), O. Balmes (b), A. Resta (b), D. Wermeille (b), R. Felici (b), S. Ferrer (b,c), and J.W. M. Frenken (a), A new role for steps in catalysis and reaction oscillations, Nature Chemistry, DOI: 10.1038/NCHEM.728.
(a) Kamerlingh Onnes Laboratory, Leiden University (The Netherlands)
(b) ESRF
(c) Present address: CELLS - ALBA, Universita Autònoma de Barcelona (Spain)
Top image: A palladium catalyst sample being heated in the UHV chamber of beamline ID03 (image courtesy R. van Rijn).