- Home
- News
- Spotlight on Science
- High-pressure synthesis...
High-pressure synthesis of polymeric silicon hydride and amorphisation and re-crystallisation of silane
17-03-2011
Polymeric silicon tetrahydride is synthesised under high-pressure from pure elements, silicon and hydrogen, that do not react with each other at ambient conditions. High-pressure studies on silane (SiH4) revealed that it does not metalise at 50 GPa as previously suggested, but instead goes through a pressure-induced amorphisation at above 60 GPa re-crystallising into a polymeric phase at around 90 GPa. Silane remains insulating up to at least 130 GPa.
Share
Scientists at the ESRF used high-pressure to synthesise SiH4 (silane) from pure elements. This material, usually made via chemical reaction using chlorine, is extensively used for industrial production of solar grade silicon with application to solar cells. It also is the principal material used in the production of polysilicon and is an essential component for thin film photovoltaics, semiconductors and LCD display manufacturing. The present study demonstrates a possibility of producing silane from solid silicon and solidified hydrogen gas at high pressures as an alternative to the chlorine route currently used in industry.
To synthesise silane, fluid hydrogen and polycrystalline silicon – elements that do not react with each other at ambient conditions – were loaded into a diamond anvil cell. Over a period of several months, pressure was increased to 123 GPa at 300 K with no chemical reaction observed. Synchrotron X-ray diffraction measurements, collected at beamline ID09A, showed a mixture of pure H2 and Si. After 8 months at 124 GPa, Raman and X-ray scattering both showed the presence of a new phase.
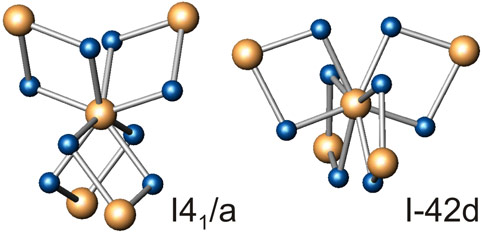
The phase formed was indexed as a body-centered tetragonal structure with lattice parameters a = 3.0864(2) Å and c = 6.957(1) Å. Silicon occupies the 4(a) position of the space group I41/a. This tetragonal phase is a product of reaction of the face-centered cubic phase of silicon with hydrogen, forming polymeric silicon tetrahydride, a phase where each silicon atom is bonded to 8 hydrogen atoms (Figure 1, left).
This tetragonal structure happens to be the same insulating phase observed following the compression of silane itself [1] as predicted from ab initio calculations for silane between 50 and 260 GPa [2]. We attempted to recover this phase, but both diamonds failed when pressure was decreased to ~100 GPa.
The rather high pressure of synthesis of silane obviously lies outside the limit for an immediate industrial application. However, we propose that the pressure and time required for this reaction to take place could be substantially reduced by varying temperature and adding catalysts to find the optimum conditions for the reaction to take place.
Recently, silane gained prominence in fundamental solid state physics and chemistry due to being suggested as a precursor to metallic hydrogen, and hence as a candidate for high-temperature superconductivity in its highly dense state [3]. Because of its high-reactivity and pyrophoric nature (silane has applications as rocket and scramjet fuel) experimental studies on silane are scarce, even being a subject of controversy [4].
We used X-ray diffraction techniques on the ID09A station to study the high-pressure behaviour of silane up to 130 GPa at room temperature as well as at 90 K using the on-line cryostat. We complemented our observation with the Raman spectroscopy measurements.
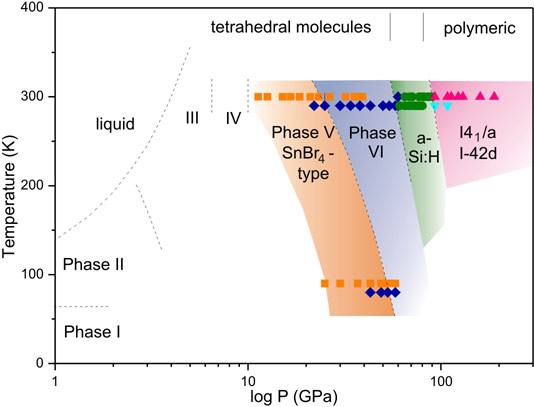
We found that silane in its SnBr4 structure (phase V) does not metallise forming a hexagonal close packed structure as it has been claimed before [1]. Instead, it transforms, via an intermediate phase-VI, to an amorphous state at above 60 GPa. At higher pressures of 90 GPa it re-crystallises into two tetragonal structures, with space groups I41/a and I-42d (Figure 1). The I41/a structure is exactly the same as observed in this work for the synthesis of silane from pure elements, as well as that observed for silane in an earlier experiment [1]. From our observations, we propose the phase diagram for silane (Figure 2) showing that the material remains insulating at least up to 130 GPa, the maximum pressure reached in this study.
 |
|
Figure 2. Proposed phase diagram of silane (logarithmic scale for pressure). |
Principal publication and authors
M. Hanfland (a), J.E. Proctor (b), C.L. Guillaume (b), O. Degtyareva (b), and E. Gregoryanz (b), High-Pressure Synthesis, Amorphization, and Decomposition of Silane, Phys. Rev. Lett. 106, 095503 (2011).
(a) ESRF
(b) University of Edinburgh, Edinburgh (UK)
References
[1] M. Eremets et al., Science 319, 1506 (2008).
[2] C.J. Pickard and R.J. Needs, Phys. Rev. Lett. 97, 045504 (2006).
[3] J. Feng et al., Phys. Rev. Lett. 96, 017006 (2006).
[4] O. Degtyareva et al., Solid State Commun. 149, 1583 (2009).