- Home
- News
- Spotlight on Science
- Photosystem II at...
Photosystem II at 3.0 Å Resolution
09-03-2006
Photosynthesis is one of the most fundamental and important natural processes. Responsible for the maintenance of the oxygen level in the atmosphere and the reduction of CO2 to carbohydrates, a detailed understanding of photosynthesis has been developed following decades of careful investigations. A complete picture of the process requires the synthesis of the information from many physical and bio-physical techniques, in addition to the three dimensional structures of the proteins involved.
Share
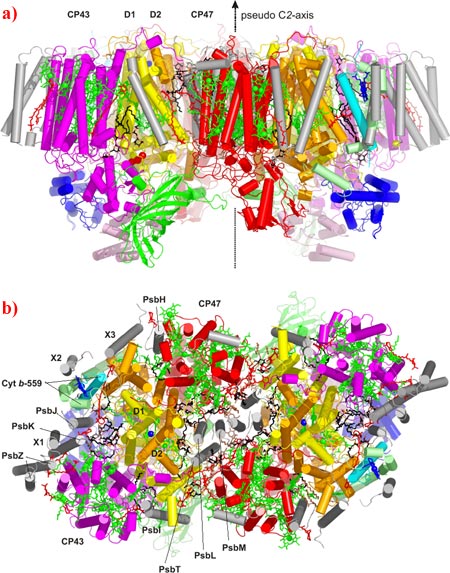
In plants, algae and cyanobacteria, photosynthesis is initiated at photosystem II (PSII), a large membrane-intrinsic homodimeric protein-cofactor complex. By capturing sunlight, PSII produces the energy required to power the oxidation of water to atmospheric oxygen. Researchers from the Freie Universität and Technische Universität of Berlin have recently been able to present the most detailed model of PSII currently available. A complex of 20 protein sub-units and 77 co-factors was revealed in near atomic detail. It seems that a high degree of flexibility is required for the protein function and that this flexibility is "lubricated" by a number of organised lipid molecules. By an appreciation of the spatial arrangement of the -carotenes and metal centres contained within PSII it was possible to further our understanding concerning the electron (and energy) transfer mechanisms required to facilitate the transfer of redox products through the components of the photosynthetic pathway.
Principal Publication and Authors
B. Loll (a,b), J. Kern (c), W. Saenger (a), A. Zouni (c) and J. Biesiadka (a), Nature 438, 1040-1044 (2005).
(a) Institut für Chemie und Biochemie/Kristallographie, Freie Universität Berlin (Germany)
(b) Present address: Max-Planck-Institut für Medizinische Forschung, Abteilung für Biomolekulare Mechanismen, Heidelberg (Germany)
(c) Institut für Chemie/Max Volmer Laboratorium für Biophysikalische Chemie, Technische Universität Berlin (Germany)