- Home
- News
- Spotlight on Science
- A different kind...
A different kind of memory in Ge-Sb-Te memory alloys
13-01-2010
Phase-change materials such as Ge-Sb-Te (GST) memory alloys are used in rewritable optical memory devices such as CDs and DVDs. In such applications, changes due to temperature are used to store data. But these materials also undergo structural transformation under pressure, with a surprising result on decompression. The structure “remembers” its initial form, either metastable cubic or stable hexagonal modification, as both exist under ambient conditions.
Share
Ge-Sb-Te (GST) alloys are the phase-change materials of choice for optical memory devices. They are also the leading candidate for a new generation of non-volatile electronic memory [1]. Data encoding is achieved by means of differences in the optical and/or electronic properties of the crystalline and amorphous phases, which can be repeatedly switched by exposure to laser or electrical pulses of different intensity and duration. A short intense pulse melts the material, which is subsequently quenched into the amorphous phase; a less intense pulse heats the material above the crystallisation temperature and reverts the process.
In real world applications, the recorded bits are subjected to significant stresses resulting from the different density of the crystalline and amorphous phases as well as volume changes due to thermal expansion. Therefore, pressure, as well as temperature, plays an important role in the functioning of such devices. Studying the response of a system to external pressure could give significant insight into the bonding characteristics of the materials involved.
GST alloys can exist in two crystalline structures: the stable phase is hexagonal, while the metastable phase that is used in optical memory devices is cubic and usually described as a distorted rock salt structure with Te forming one face-centred cubic (fcc) sublattice and Ge, Sb atoms and vacancies forming the other fcc sublattice. In our initial study [2], we demonstrated that cubic GST alloys become amorphous under pressure, which opened up a new perspective on the phase-change process. This process is general for the cubic phase of GST alloys of different compositions [3] and is not temperature-activated [4].
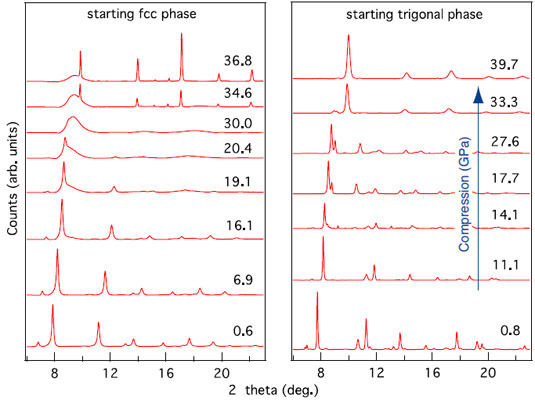
Here we focus on the different responses to pressure/decompression of the starting hexagonal and cubic phases. Figure 1 shows the evolution of the diffraction pattern of the two phases upon compression. The cubic phase becomes amorphous at pressures exceeding 15 GPa and eventually recrystallises into a bcc phase at 30 GPa. The stable hexagonal phase first changes to an orthorhombic structure and subsequently also to the bcc phase.
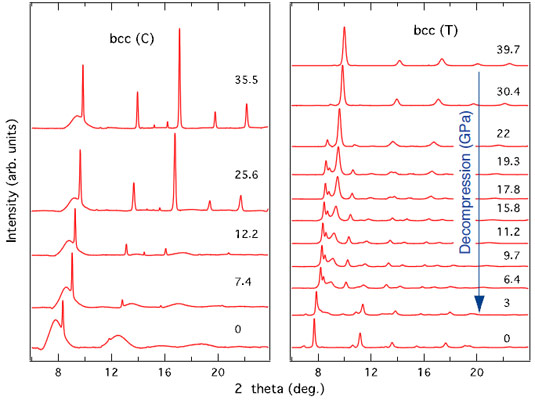
Interestingly, decompression of the bcc phase exhibits a memory of the initial structure: while the bcc phase obtained from the cubic phase becomes amorphous upon decompression, the bcc phase obtained from the stable hexagonal phase goes back to the stable hexagonal phase (Figure 2).
The memory of the initial structure is explained by the presence of vacancies in the cubic phase (and their absence in the hexagonal phase). The presence of vacancies allows large atomic displacements and phase separation and as a consequence the material “forgets” its initial structure. In the denser hexagonal phase, the atomic displacements are on a smaller scale and the material retains the memory of its initial structure.
References
[1] M. Wuttig, N. Yamada, Nature Mater. 6, 824 (2007).
[2] A.V. Kolobov et al., Phys. Rev. Lett. 97, 035701 (2006).
[3] A.V. Kolobov et al., Appl. Phys. Lett. 91, 021911 (2007).
[4] M. Krbal et al., Appl. Phys. Lett. 93, 031918 (2008).
Principal publication and authors
Initial structure memory of pressure-induced changes in the phase-change memory alloy Ge2Sb2Te5, M. Krbal (a,b), A.V. Kolobov (b), J. Haines (a), P. Fons (b), C. Levelut (a), R. LeParc (a), M. Hanfland (c), J. Tominaga (a), A. Pradel (a), M. Ribes (a), Phys. Rev. Lett. 103, 115502 (2009).
(a) University of Montpellier, Montpellier (France)
(b) National Institute of Advanced Industrial Science and Technology, Tsukuba (Japan)
(c) ESRF, Grenoble (France)