- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2007
- Materials Science
- Natural gas hydrates investigated by X-ray synchrotron diffraction
Natural gas hydrates investigated by X-ray synchrotron diffraction
Gas-hydrates (clathrates) are crystalline inclusion compounds that occur naturally on Earth, where suitable conditions of high pressure and low temperature prevail. These conditions are encountered in marine sediments mainly offshore along continental margins, and to a lesser extent in polar regions (permafrost). Methane is present in huge amounts in these hydrates, estimated to 10000 Gt, which exceeds by far the other known fossil energy sources of coal, oil and gas. They will be of major economic interest in the future once techniques for their safe extraction are established. Consequently, natural gas hydrates continue to attract increasing attention from the gas industry because of their potential as an energy resource. In contrast to their potential benefits to mankind, their role has been repeatedly quoted in geological hazards, or global climate change. Different scenarios explain the possible role of hydrates in past and future global climate change because of the potentially enormous greenhouse feedback effect of released methane. Information about their occurrence in natural environments is of prime importance in order to establish relationships between climate change and gas hydrates, to elucidate the mechanism of gas hydrates’ involvement in geo-hazards or to exploit the natural gas from hydrates profitably. This supposes a knowledge of how gas hydrates exists in the natural environment, their structural type, composition, their stability fields (p-T), etc.
We have used X-ray synchrotron diffraction to investigate the structure and thermal properties of natural samples. Gas hydrates have been recovered during the ZAIANGO cruise (ZAIROV-Leg 2 Dec. 2000, TOTAL - IFREMER) by gravity coring at 3160 m water depth in the Congo-Angola basin [1]. Other specimens were retrieved during the NERIS II campaign (Dec. 2004) at water depths between 1147 and 1203 m in the deep province of the Niger delta (Gulf of Guinea) (Figure 41). The powder data were recorded at beamline ID31 which allowed high-quality powder-diffraction patterns to be obtained with a typical resolution of ![]() d/d ~ 10-4. The physical (structure) and chemical properties of the gas hydrates can be preserved at low temperature (~ 90 K) and the thermal properties can be studied under controlled conditions.
d/d ~ 10-4. The physical (structure) and chemical properties of the gas hydrates can be preserved at low temperature (~ 90 K) and the thermal properties can be studied under controlled conditions.
 |
|
Fig. 41: a) Natural gas hydrate from the African margin decomposing at atmospheric conditions; b) Microscopic view of the gas hydrates from African margin preserved at 113 K. |
The natural gas hydrates consist of mixed crystalline phases: ice Ih (hexagonal) and a cubic lattice structure I (sI), which is the most common structure adopted by natural specimens. Hydrocarbon gases in hydrates originate generally from two sources: biogenic and thermogenic. Our previous study using different laboratory techniques confirmed that the presence of sI is associated with methane (the major gas component) originating from microbial CO2 reduction [1]. Hydrates of thermogenic origin (generally composed of higher hydrocarbons) are generated at high temperature in the deep sediment at ~ 1000 to 2000 m (or even deeper) below the sea floor. Their structure generally differs from sI. However, they can also form near the sea floor when associated with fluid and seepage.
Figure 42 displays the lattice constants of the Congo-Angola and Niger specimens (a = 11.8646 (39) Å and a = 11.8619 (23) Å, respectively) at ~ 90 K [2]. They are comparable but not identical to those obtained for sI natural hydrates from Cascadia margin in the Pacific, Okhotsk Sea and synthetic methane hydrates [3]. The differences in lattice constants between natural specimens collected at different geological sites may reflect the mixing of methane with components like H2S, CO2 and non-methane gases identified spectroscopically in the structure [4].
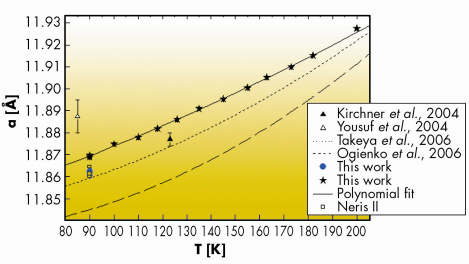 |
|
Fig. 42: Lattice expansion of gas hydrates as a function of temperature. |
Another point investigated here is the thermal evolution of the lattice expansion. Gas hydrates are known to present unusual thermophysical properties when compared to ice. They appear to depend on the interactions of guests molecules with the water host lattice. We confirmed that the thermal expansivity is much larger than that of ice, especially below 200 K. Furthermore, it tends to become reduced as temperature increases up to the hydrate decomposition temperature [2]. Conversely, at lower temperature, the thermal expansivity is much larger than compared to ice. This is attributed to a perturbation of the vibrational motions of the water molecules that experience a large anharmonic potential due to strong interaction with the guests.
In conclusion, valuable structural information can be obtained on well-preserved natural hydrates. We suggest that non-methane gas components present in small proportions affect the lattice dimension of the sI structure to a small extent. Moreover, the derived thermal expansion coefficients show comparable values with those reported for synthetic methane hydrates at low temperature and tends to reach thermal expansion of ice at higher temperature.
Acknowledgements
We thank the companies TOTAL and IFREMER for co-funding and gas hydrates collection during the joint projects ZAIANGO and NERIS II. Technical support from the ESRF is greatly appreciated.
References
[1] Charlou et al., Chem Geol. 205, 405 (2004).
[2] Bourry et al., Geophys. Res. Lett. 34, L22303 (2007).
[3] Yousuf et al., Appl. Phys. A 78, 925-939 (2004); Takeya et al., Chem. Eng. Sci. 61, 2670-2674 (2006); Ogienko et al., J. Phys. Chem. B. 110, 2840-2846 (2006).
[4] Chazallon et al., Chem. Geol. 244, 175 (2007).
Authors
B.Chazallon (a), C. Bourry (b), J.L. Charlou (b), J.P. Donval (b), M. Brunelli (c), C. Focsa (a)
(a) PhLAM, UMR CNRS 8523, FR-CNRS 2416, University Lille 1 (France).
(b) Géochimie et Métallogénie, Géosciences Marines, IFREMER Brest (France)
(c) ESRF



