- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2007
- Materials Science
- Unusual microstructure modification in biogenic crystals under annealing
Unusual microstructure modification in biogenic crystals under annealing
Biogenic crystals are crystals produced by living organisms. They attract a lot of attention because of their fascinating microstructures and superior properties [1]. The interrelation between a material’s microstructure and its properties is of great interest for materials science and engineering. Impressive examples include our ability to control the strength of metals and alloys by annealing-mediated grain size, and band gap engineering in semiconductor thin films by lattice-mismatch-mediated interfacial strains. Unique methods of controlling microstructure and, hence, crystal properties also exist in nature. In fact, organisms can control the polymorphism, morphology, and orientation of crystalline blocks in biogenic crystals by means of organic macromolecules deeply involved in the biomineralisation process. Very recently it was shown that even the atomic structure of biogenic ceramics can be slightly different when compared to the structure of their geological counterparts [2]. In fact, the aragonite and calcite polymorphs of calcium carbonate extracted from various mollusc shells exhibited anisotropic distortions of the crystalline lattice of about 0.1–0.2% [3], which are rather high for ceramic materials. These distortions (lattice strains) are effectively relieved at surprisingly low annealing temperatures of about 150–200°C.
In continuation of this work at the high-resolution powder diffraction beamline, ID31, we unexpectedly found that strain relaxation is accompanied by a remarkable broadening of the X-ray diffraction profiles, an effect easily visible by eye. By fitting the measured diffraction profiles to a Voigt function and separating the Lorentzian- and Gaussian-type contributions to the diffraction peak broadening, we were able to extract the crystallite size and averaged microstrain fluctuations as functions of annealing temperature.
 |
|
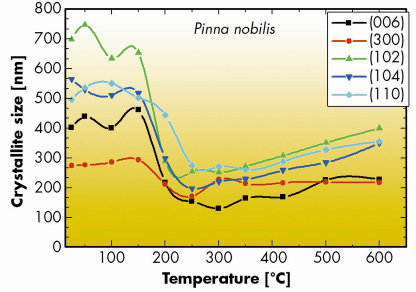
Fig. 43: Anisotropic crystallite sizes along different crystallographic directions in calcitic shells of Pinna nobilis measured as a function of annealing temperature. Prior to diffraction measurements at room temperature, the shell powders were annealed for 30 minutes at temperatures between 50 and 600°C. |
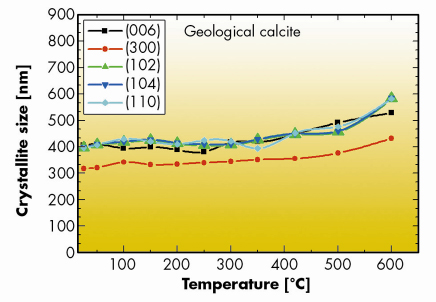
The analysis revealed a considerable reduction in the crystallite size in biogenic calcite and aragonite annealed at temperatures above 150–200°C. Typical results are plotted in Figure 43. The crystallite size reduction correlates with a growth in the averaged microstrain fluctuations (not shown here) caused by the increased number of inter-crystallite boundaries, which are the sources of the inhomogeneous deformation fields [4]. The reduction of crystallite sizes (by a factor of three in some cases) with annealing temperature is very unusual for ceramic materials in which thermally-activated grain growth is regularly observed under annealing. The latter behaviour is thermodynamically justified since it reflects the tendency of the system to reduce free energy by diminishing the number of grain boundaries. In fact, we did observe a gentle grain growth in this type of sample with geological calcite, heat treated up to 600°C (see Figure 44). Geological aragonite did not reveal any changes in crystallite size under annealing at temperatures up to 400°C.
 |
|
Fig. 44: Anisotropic crystallite sizes along different crystallographic directions in geological calcite (Creel, Chihuahua, Mexico) measured as a function of annealing temperature. Experimental condition and preparation were the same as for calcite shells (Figure 43). |
Based on these findings, we assumed that intra-crystalline organic macromolecules trapped within individual crystallites of calcium carbonate are responsible for the observed structural modifications. According to our model considerations, the network of organic macromolecules supplied by an organism assist in the nucleation of calcium carbonate at an early stage of biomineralisation. There is evidence that biogenic calcium carbonate initially grows as amorphous. Crystallisation of amorphous calcium carbonate within the confined space defined by the macromolecular network increases the forces imposed by organic macromolecules on the mineral lattice because of the difference in specific volumes per molecule in the amorphous and crystalline phases. These forces cause lattice distortions revealed in our high-resolution diffraction measurements.
Annealing at 150–200°C leads to degradation of the organic macromolecules, which initially support the strained mineral lattice. As a consequence, lattice strains are effectively relieved. Besides this, defragmentation of organic macromolecules modifies the topology of the connected crystalline units and, in particular, introduces angular misorientation between them, which is revealed as the reduction in the size of the coherence length of the crystalline blocks.
The high quality experimental data and the model developed provide new insight into the interactions between the organic and ceramic components in biogenic crystals. We believe that a deeper understanding of this interaction will facilitate future development of bio-inspired advanced materials.
References
[1] S. Weiner, L. Addadi, J. Mater. Chem. 7, 689 (1997).
[2] B. Pokroy, J.P. Quintana, E.N. Caspi, A. Berner, E. Zolotoyabko, Nature Mater. 3, 900 (2004).
[3] B. Pokroy, A. Fitch, P. Lee, J.P. Quintana, E.N. Caspi, E. Zolotoyabko, J. Struct. Biol. 153, 145 (2006); B. Pokroy et al., J. Struct. Biol. 155, 96 (2006).
[4] E. Zolotoyabko, J.P. Quintana, J. Appl. Cryst. 35, 594 (2002).
Principal publication and authors
B. Pokroy (a), A. Fitch (b), E. Zolotoyabko (a), Advanced Materials 18, 2363 (2006); Crystal Growth & Design 7, 1580 (2007).
(a) Department of Materials Engineering, Technion, Haifa (Israel)
(b) ESRF



