- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2013
- Electronic structure and magnetism
- Production of Ru nanoparticles over activated carbons: chemical interactions of the metal precursor with surface functional groups
Production of Ru nanoparticles over activated carbons: chemical interactions of the metal precursor with surface functional groups
Carbon–based materials such as activated carbon, nanotubes, graphene and fibres are gaining interest as supports for catalytic materials or as catalysts themselves. These types of carbon-based materials are also promising targets for the production of a new generation of heterogeneous catalysts, which are required for bio-refining raw organic substrates obtained from the biomass [1]. Therefore, the preparation of metallic nanoparticles anchored to carbon surfaces is at present an important technological topic. But many basic aspects of the preparation-activation procedures for synthesising these catalysts are still not well established. For instance activated carbon (AC) surfaces are usually considered as inert material; however the presence of oxygen surface groups (chemical species such as carboxyl acids, hydroxyls, carbonyls and lactones) can be decisive for the anchoring of metallic precursors as well as for the generation of nanoparticles during the reduction-activation treatments. These surface groups can also play a decisive role in the final catalytic site structures, and consequently in their catalytic properties. They can affect the electronic properties of the metallic nanoparticles [2] and they can be destabilising for metallic aggregates and sintering phenomena. In this latter case, the desorption of oxygen surface groups during the decomposition/reduction of the metal precursor can also lead to surface reconstructions of the metal nanoparticles during the starting-up of a catalytic material for a given process.
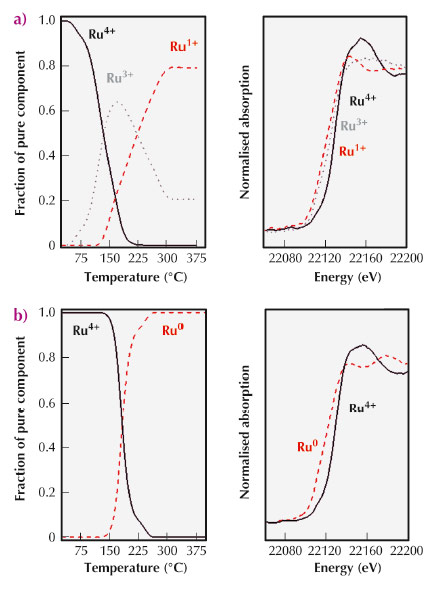 |
|
Fig. 95: Concentration profiles obtained from Ru K-edge XANES spectra of the different Ru species (Ru+4, Ru+3, Ru+1 and Ru0) observed during TPR of Ru(NO)(NO3)3 precursor supported on AC-ox (a) and AC-f (b). |
To shed light on these surface chemical phenomena taking place over the carbon surfaces by solid-solid reactions with the metal precursor, we have previously performed studies including temperature programmed reduction (TPR), characterisation of the final surface properties of the generated metal nanoparticles, including detection of electronic properties by CO chemisorptions coupled with calorimetry, and determination of catalytic properties in several test reactions. An important conclusion from these studies is that we need to pursue the evolution of the chemical species in the solids in situ, instead of determining the final states of the metal nanoparticles a posteriori, in order to understand the genesis of the metallic nanoparticles. Thus, we want to evaluate the chemical processes from the point of view of the solids (carbon support and metal precursor) which evolve during catalyst activation. We have already been able to follow the progress of the oxidation states of Ru atoms when a Ru(NO)(NO3)3 complex is reductively decomposed over AC surfaces. In fact, we have comparatively studied the interaction of this inorganic catalytic precursor over two activated carbons, one thermally treated to remove all oxygen groups (AC-f) and the second that is the as-received material (AC-ox), where the surface contains significant amounts of oxygen functional groups. Note that these oxygen surface groups can also be produced at the initial steps of the metallic precursor decomposition [3]. Performing an in situ XANES analysis of the Ru K-edge at beamline ID24, we tracked the behaviour of Ru precursors under a hydrogen atmosphere and analysed the reduction mechanism as a function of the surface state of the AC support (Figure 95). In particular, we found that oxygen groups exposed on the AC-ox surfaces generate a multi-step mechanism for the reduction of the Ru species. In the case of clean graphitic surfaces (AC-f), a single step was observed.
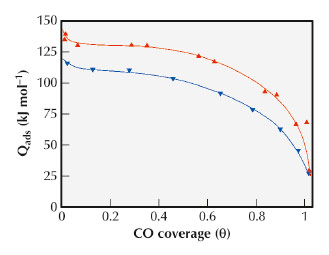 |
|
Fig. 96: Differential heats of CO adsorption as a function of the surface coverage (Δ) 2%Ru/AC-ox and (∇) 2%Ru/AC-f catalysts. |
This study may thus reveal details of the specifity of the carbon-nanometal interaction that could be important when these materials are used as catalysts. Figure 96 displays the significant differences in electronic properties that were detected by microcalorimetry of CO chemisorptions, when the exposed surface metallic sites (Ru) are supported on ACs, with or without initial oxygen functionalities. Note that these materials present a similar Ru primary particle size. These interactions have a significant consequence on the catalytic properties. Ru nanoparticles generated on the AC-f exhibit five times higher catalytic activity than when supported on AC-ox for the ammonia decomposition reaction.
Principal publication and authors
F.R. García-García (a), M. Fernández-García (b), M.A. Newton (c), I. Rodríguez-Ramos (b) and A. Guerrero-Ruiz (a), ChemCatChem 5, 2446–2452 (2013).
(a) Facultad de Ciencias, UNED, Madrid (Spain)
(b) Instituto de Catálisis y Petroleoquímica, CSIC, Madrid (Spain)
(c) ESRF
References
[1] P. Gallezot, Chem. Soc. Rev. 41, 1538–1558 (2012).
[2] F.R. García-García, J. Álvarez-Rodríguez, I. Rodríguez-Ramos and A. Guerrero-Ruiz, Carbon 48, 267-276 (2010).
[3] E. Gallegos-Suarez, M. Perez-Cadenas, A. Guerrero-Ruiz, I. Rodríguez-Ramos and A. Arcoya, Appl. Surf. Sci. 287, 108-116 (2013).



