- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2013
- Electronic structure and magnetism
- Defect clusters of lanthanide ions in luminescent materials
Defect clusters of lanthanide ions in luminescent materials
Phosphor converted light emitting diodes (pc-LEDs) have a much lower energy consumption and a longer lifetime than traditional light sources, such as incandescent lamps, and are quickly taking over the market of general lighting. Usually a pc-LED is a combination of a blue emitting LED and a yellow emitting luminescent material (or phosphor). The overall emission spectrum of this combination often lacks sufficient output in the red part of the spectrum and as such the colour rendering is poor. This has encouraged the research into materials for LED applications showing a saturated red emission.
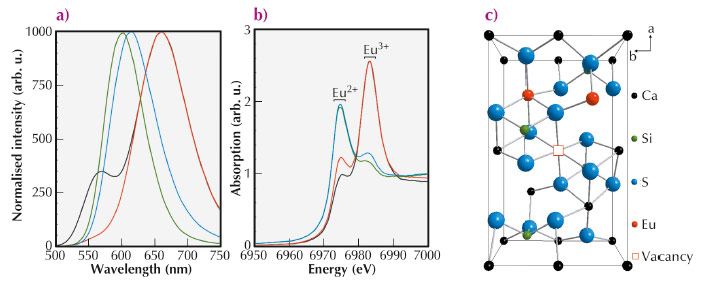
Europium doped Ca2SiS4 is an interesting candidate due to its bright yellow-to-red emission [1]. While samples with low Eu dopant concentrations (< 15%) crystallise in the orthorhombic structure of Ca2SiS4, highly doped samples ( > 50%) have the monoclinic structure of Eu2SiS4. The dopant concentration also strongly affects the emission characteristics (Figure 99a). At low dopant concentrations, the emission spectrum consists of two broad bands (564 nm and 660 nm) due to the presence of two inequivalent lattice sites for the Eu ions. At higher concentrations, only a single band is visible which linearly shifts from 614 nm to 577 nm upon increasing Eu concentration.
The emission of Eu2+ ions is highly dependent on their local environment. To study this local environment, we performed X-ray absorption near edge (XANES) measurements at the Eu L3 edge at BM26A and extended X-ray absorption fine structure (EXAFS) measurements at the Eu K edge at BM23 [2].
The measured europium L3 edge XANES spectra are shown in Figure 99b, exhibiting two strong white lines. These originate from the coexistence of the two common valence states of the Eu ions (Eu2+ and Eu3+). While the Eu ions are mainly present in a trivalent state for the lightly doped samples [3], divalent Eu is dominant in the highly doped samples.
The local structure around the Eu ions was addressed by Eu K edge EXAFS. For all samples, the Eu occupancy of both sites in the structure was investigated. The coexistence of both valence states was implemented in the simulations in the lightly doped samples, while only the presence of Eu2+ was modelled in the highly doped samples.
Firstly, lets consider the low dopant concentrations. In earlier work it was reported that the Eu ions occupy both inequivalent Ca sites in the Ca2SiS4 host crystal, and this was confirmed by the EXAFS data. Most of the Eu3+ ions substitute on the Ca2 site, while the divalent ions mainly occupy the Ca1 site. Upon increasing the dopant concentration the relative occupancy of the Ca1 site increases. This agrees well with the optical data: upon increasing the dopant concentration the relative intensity of the emission band caused by the Ca1 site (at 660 nm[4]) increases.
The presence of trivalent Eu ions on divalent Ca sites can cause disorder and structural defects in the samples. Using EXAFS we were able to prove that in the sample with a dopant concentration of 2% no such defects are present. For the more heavily doped samples, i.e. 7.5% and 10%, the following defects were found from EXAFS model fitting: (a) One trivalent Eu ion at a Ca2 site, and a vacancy and the other Eu3+ ion at a Ca1 site; (b) Both trivalent ions on a Ca2 site, and a vacancy appearing at a Ca1 site (Figure 99c).
 |
|
Fig. 99: (a) Photoluminescence emission spectra of Eu doped Ca2SiS4 with different dopant concentrations (excitation wavelength = 450 nm). (b) Eu L3 XANES spectra of Eu doped Ca2SiS4 with different dopant concentrations. For both figures: (black) 2 molar%, (red) 10 molar%, (blue) 50 molar% and (green) 75 molar %. (c) In the lightly doped samples defect clusters are observed; one of these defects is represented schematically. |
In contrast, for the high dopant concentrations, the structure of highly doped samples can be described as Ca ions substituting for Eu ions in a Eu2SiS4 crystal. The EXAFS analysis shows that the Ca ions preferentially occupy the Eu2 site in the lattice. This preferential distribution decreases with an increase in Eu concentration until an equal distribution of the Eu ions in Eu2SiS4 is obtained.
Based on these results we could explain the concentration dependence of the emission spectra of (Ca,Eu)2SiS4. At low Eu concentrations (< 10%) the dopant ions substitute for the Ca ions at both inequivalent crystallographic sites, albeit that some preferential substitution is observed. At higher concentrations (> 40%) the Ca ions occupy both europium sites in the monoclinic Eu2SiS4 structure. Furthermore an unexpected large fraction of trivalent europium ions is found in the XANES spectra of lightly doped thiosilicates, although no Eu3+ emission is found in the photoluminescence spectra.
Principal publication and authors
K. Korthout, A.B. Parmentier, P.F. Smet and D. Poelman, Phys. Chem. Chem. Phys., 15, 8678-8683 (2013).
LumiLab, Department of Solid State Sciences, Ghent University (Belgium)
References
[1] P.F. Smet, N. Avci, B. Loos, J.E. Van Haecke and D. Poelman, J. Phys.: Condens. Matter 19, 246223 (2007).
[2] J.J. Joos, K. Korthout, S. Nikitenko, D. Poelman and P.F. Smet, Opt. Mater. Express 3, 1338-1350 (2013).
[3] K. Korthout, K. Van den Eeckhout, J. Botterman, S. Nikitenko, D. Poelman and P.F. Smet, Phys. Rev. B 84, 085140 (2011).
[4] A.B. Parmentier, P.F. Smet and D. Poelman, Opt. Mater. 33, 141–144 (2010).



