- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2014
- Enabling technologies
- High pressure cooling of biological crystals without cryoprotection
High pressure cooling of biological crystals without cryoprotection
Most diffraction data for biological crystals is recorded at cryogenic temperatures to reduce the secondary radiation damages and thereby extend the life-time of samples in X-rays. However, crystals need to be cryo-protected prior to being cryo-cooled to avoid solvent crystallisation and its subsequent spoiling of the experimental results: the loss of diffraction orders and of diffraction quality. To a lesser extent, the cryo-protection process also has its own undesirable effects: (1) crystals are slightly destabilised during the short soaking passage in the cryosolution and extremely fragile crystals may dissolve regardless of the cryo-agent type, (2) the vitrification of solvent into low density amorphous (LDA) ice at ambient pressure produces a mechanical stress that increases the mosaicity of crystals, and (3) cryo-molecules may bind and disturb proteins possibly even at their active sites. The search for optimum cryoprotectant conditions which aim to limit these drawbacks is so time-consuming for usually a poor improvement that crystallographers often prefer to simply use glycerol. Therefore, we investigated alternative cooling methods to eliminate the need for cryoprotection in an attempt to circumvent this issue. High pressure cooling is one of the most effective of these techniques and consists of flash-cooling cryoprotectant-free biological crystals down to 77 K under 2 kbar of helium pressure (Figure 148a) [1]. During this thermodynamical process, the crystals are frozen in their non-protected mother liquor which is directly transformed into high density amorphous (HDA) ice under the effect of pressure, avoiding water crystallisation and its destructive effects. Advantageously, the cryoprotectant-free HDA matrix limits the augmentation of the mosaicity which results from the LDA-ice expansion, eliminating the unwanted effects that are associated with the presence of cryo-agents, and thus preserves crystals close to their native state. High pressure cooling has proven to be systematically applicable to all kinds of biological crystals regardless of the protein’s nature, the solvent content or the crystallisation conditions. Moreover, the method has recently been extended to cryopreserve hydrated bacteria for imaging experiments [2]. In addition, pressure is a key thermodynamic parameter as important as temperature in studies of macromolecules. Indeed, pressure allows the energy landscape of proteins to be explored, providing access to different conformational substates that cannot be studied in any other manner (active site conformations or reaction coordinates). Therefore, this technique has potential to address many essential biological issues.
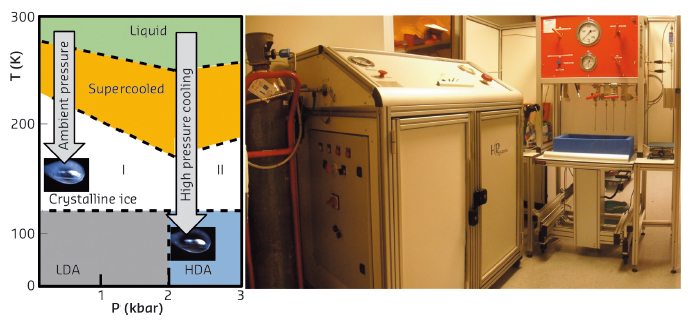 |
|
Fig. 148: a) Phase diagram of water. Unprotected crystals cooled at ambient pressure (AP-cooled) produce crystalline ice. At high pressure the solvent is transformed into HDA-ice. b) High pressure cooling installation at beamline ID23. |
Until now, users have been put off by this difficult approach reserved for specialists, which needs heavy instruments of low productivity to combine high pressures with cryogenic temperatures. We have designed an automated high pressure cooling instrument and developed a concomitant methodology and ancillary equipment, paying particular attention to the user-friendliness and throughput (Figure 148b). The computer controlled system is theoretically capable of processing 30 samples per hour. The throughput bottleneck now mainly depends on classical sample handling operations of crystallographers facilitated by a new pluggable Spine compatible sample-holder and its associated toolkit (i.e. crystal fishing, recovery and mounting/dismounting). Optionally, during the high pressure cooling procedure, the pressurisation bench can be switched to a noble gas circuit to produce xenon or krypton heavy atom derivatives for anomalous phasing purposes. In practice, the machine is capable of vitrifying nanolitres of aqueous solution with only 5% glycerol for cryopreserving dilute biological objects. Moreover, the method is particularly effective for cooling biological crystals that are totally free of cryoprotectant since the ingredients of the mother liquor are sufficient to act as antifreeze agents. The methodology was successfully validated with a series of test crystals (lysozyme, insulin, thaumathin, FEA, proteinase-K and thermolysin). Following this, nearly a thousand users’ crystals have been processed. The resulting structures are relatively isomorphous to their counterparts in the PDB, since the pressure induced structural changes are limited to flexible loops at the proteins surfaces while their structured cores are relatively pressure insensitive. High pressure cooled FAE is a meaningful example, revealing a pressure induced modification that concerns only a single loop at the dimer interface, but this simple modification results surprisingly in a superior crystalline quality: a transition to a higher symmetry, a higher resolution, lower B-factors and a lower mosaicity (Figure 149).
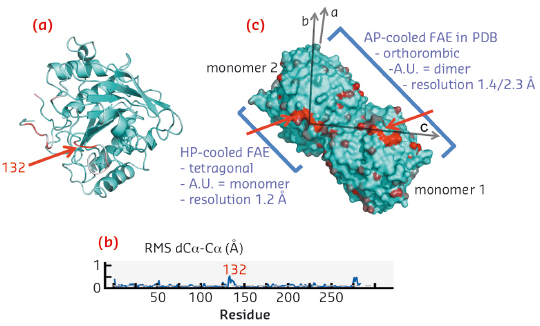 |
|
Fig. 149: a) Ribbon representation of the structure of the HP-cooled protein FEA, largest structural displacements are represented in red (loop 132). b) Root mean square displacement of the HP-cooled FEA versus residue number. c) Crystallographic packing of FAE in surface representation (monomer/dimer). |
The apparatus is installed in the laboratory associated with beamline ID23, and a high pressure cooling service is now offered to the ESRF structural biology user community for appropriate projects.
Principal publication and authors
P. van der Linden, F. Dobias, H. Vitoux, U. Kapp, J. Jacobs, S. Mc Sweeney, C. Mueller-Dieckmann and P. Carpentier, J. Appl. Cryst. 47, 584 (2014).
ESRF
References
[1] C.U. Kim, R. Kapfer and S.M. Gruner, Acta Cryst. D 61, 881 (2005).
[2] E. Lima, Y. Chushkin, P. van der Linden, C. Un Kim, F. Zontone, P. Carpentier, S.M. Gruner, and P. Pernot, Phys. Rev. E 90, 042713 (2014).



