- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2014
- Structural biology
- Structure of a mammalian serotonin receptor revealed by X-ray crystallography
Structure of a mammalian serotonin receptor revealed by X-ray crystallography
The complex mental capacities and motor behaviours of mammals rely on the evolution of nervous systems in which ligand-gated ion channels facilitate fast intercellular signalling. The Cys-loop receptor family encompasses pentameric ion channels such as the nicotinic acetylcholine and serotonin 5-HT3 (resp. GABA and Glycine) receptors which are cation- (resp. anion-) selective and promote excitatory (resp. inhibitory) signals. These receptors, targets of many psycho-active and therapeutic compounds, function as allosteric signal transducers whose conformational transitions, regulated by neurotransmitter binding, operate the gating of a channel that let ions flow through the plasma membrane. Each of their five subunits contains three domains: a β-sheet-rich extracellular domain comprising the ligand-binding site, an α-helical transmembrane domain made of helices named M1 to M4, and an intracellular domain, a major determinant of gating kinetics and channel conductance. Even though structural knowledge of these receptors is rapidly increasing (see e.g. [1-2]), the latter domain, specific to metazoa, is absent in crystal structures derived thus far.
To further document the mechanistic details of the functional processes of pentameric ligand-gated ion channels, high-resolution structures of full-length mammalian receptors have to be solved. In an effort toward this goal, we crystallised a mouse homopentameric 5-HT3A receptor that retains a large part of its intracellular domain. The full-length receptor was expressed in HEK 293 cells [3]. First attempts yielded crystals diffracting X-rays only to 20 Å resolution and limited proteolysis experiments improved this limit to 7 Å. We eventually found that the Llama-derived single-chain antibody VHH15 formed a stable complex with the 5-HT3 receptor and crystals of this complex diffracted to 3.5 Å resolution at beamline ID29.
 |
|
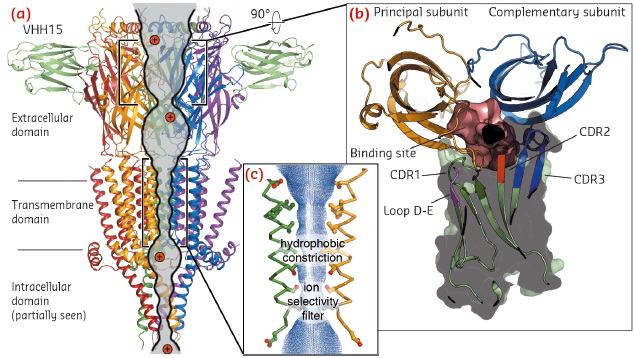
Fig. 104: a) View parallel to the membrane plane of the crystal structure of the split 5-HT3A receptor in complex with VHH15 in cartoon representation. Two out of the five VHH15 molecules observed are shown in pale green. The ion (pictured in red) permeation pathway is shown as a continuous solvent accessible volume (grey). b) Rotated view of the interface between two neighbouring extracellular domains and the solvent accessible volume (pink) representing the serotonin binding site. One VHH15 molecule (hidden in the main panel) is also displayed in cartoon and surface representation, highlighting the extensive interaction between its loops and the ligand-binding site. c) Close up of the transmembrane domain pore. |
In these crystals, the 5-HT3 receptor subunits are arranged in pseudo five-fold symmetry around a central ion pathway perpendicular to the membrane plane (Figure 104a). The extracellular domain reveals the detailed anatomy of the serotonin binding-site capped by a VHH15 bound radially to the subunit interface (Figure 104b). The transmembrane domain delimits an aqueous pore lined by the M2 helices (Figure 104c), whose state (open or closed) cannot be unambiguously defined without more dynamical information.
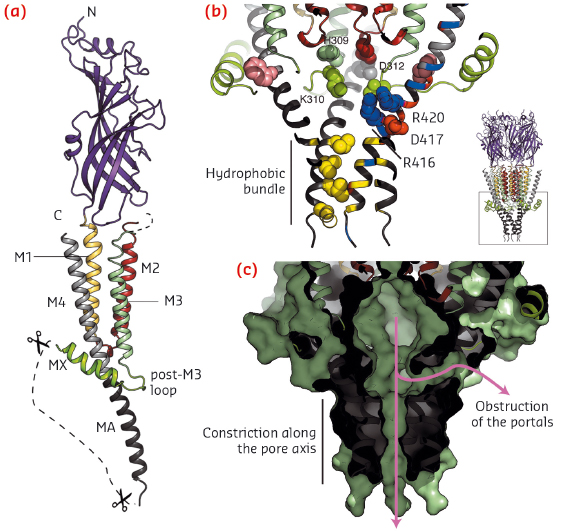
A portion of the intracellular domain structure is revealed in the crystal structure (Figure 105a): the post-M3 loop and the short MX helix clamp the helix MA, the latter a continuous extension of M4, and create a vestibule exposing charged residues on its inner surface that were identified as determinants of single channel conductance by mutagenesis studies (Figure 105b). Here, lateral ion pathways that might be formed between MA helices are blocked by the post-M3 loop and, further down, the MA helical hydrophobic bundle tightens, thus also constricting a possible exit pathway for ions (Figure 105c). Again, a more dynamical view of the receptor would be needed to gain further insight into ion permeation mechanism through the intracellular vestibule. The missing digested segment, between MX and MA, contains interaction sites for proteins modulating activity, assembly, trafficking and clustering.
 |
|
Fig. 105: a) View of one subunit of the split 5-HT3A receptor shown in cartoon representation. b) The intracellular domain. Key residues are shown as spheres. c) Same view, in surface representation, highlighting the vestibule and the two constricted ion exit pathways. |
The structure of the split 5-HT3 receptor shows a number of interesting features. Firstly, it reveals part of the intracellular domain, thus expanding the structural basis for understanding the operating mechanism of mammalian Cys-loop receptors. Secondly, our finding that a particular nanobody binds at the subunits’ extracellular interface opens the possibility to specifically target Cys-loop receptor subtypes: a crucial challenge in developing new active compounds and in determining the crystal structures of pentameric ion channels.
Principal publication and authors
G. Hassaine (a,b), C. Deluz (a), L. Grasso (a), R. Wyss (a), M.B. Tol (a), R. Hovius (a), A. Graff (c), H. Stahlberg (c), T. Tomizaki (d), A. Desmyter (e), C. Moreau (f), X.-D. Li (g), F. Poitevin (h), H. Vogel (a) and H. Nury (a,f), Nature 512, 276-81 (2014).
(a) LCPPM, EPFL, Lausanne (Switzerland)
(b) Present address: Theranyx, Marseille (France)
(c) CCIN, Biozentrum, University of Basel (Switzerland)
(d) SLS, PSI, Villigen (Switzerland)
(e) AFMB, CNRS & Université Aix-Marseille, Marseille (France)
(f) Institut de Biologie Structurale, Grenoble (France)
(g) LBR, PSI, Villigen (Switzerland)
(h) DSM, Institut Pasteur & CNRS, Paris (France)
References
[1] L. Sauguet et al., PNAS 111, 966-71 (2014).
[2] P. Miller and A.R. Aricescu, Nature 512, 270-5 (2014). [3] G. Hassaine et al., Biochim. Biophys. Acta 1828, 2544-52 (2013).



