- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2015
- Structure of materials
- Evidence of the impact of solvated nanoparticles upon their surroundings
Evidence of the impact of solvated nanoparticles upon their surroundings
Colloidal nanoparticles are widely used in dispersed form for consumer and industrial goods. However, little is known about the chemical environment near the particle surface. Using high-energy X-ray scattering, it has now been shown that nanoparticles subtly influence the local solvent density. Each nanoparticle induces a nanoscopic solvation shell, reaching out as far as 2 nm from the particle surface. This effect is expected to strongly influence the chemistry and rheology of these important materials.
The properties of matter at the nanoscale can differ wildly from that of bulk materials. For example, pronounced increases in chemical reactivity, or changes in optical properties are commonplace. In recent years, nanoparticles with a typical size of 1-100 nm have therefore been used in a wide diversity of applications. In many real world applications, for example in sun screen, a liquid or gel carrier is used. To prevent agglomeration and so prolong the lifetime of the product, it is important to know how the solvent interacts with the nanoparticles. Likewise, in homogeneous catalysis, where chemical reactions occur at the particle surface, it is important to know whether the local chemical environment is different from that of the bulk solvent.
The reorganisation of solvent molecules around isolated cations has been recognised for many years [1]. Pioneering experiments with synchrotron radiation have shown how bulk planar surfaces can induce surface ordering in fluids [2]. However, no definitive proof previously existed for the formation of a nanoscale solvation shell around colloidal nanoparticles. Nevertheless, such effects have been reported in simulations of solvated nanoparticles [3]. In real systems, this would influence properties including diffusion coefficients and particle growth, which influence the applications described above.
The influence of solvated nanoparticles upon their surroundings has been addressed experimentally using high-energy X-ray scattering at beamline ID15B (now ID15A), in particular using the pair distribution function (PDF) technique. This technique makes use of area detectors to collect scattering data up to a very high resolution in reciprocal space. Fourier transformation then yields the PDF, which is a histogram of all the interatomic distances in the material. Well-defined species, such as the ZnO nanoparticles studied here, yield sharp peaks whereas the isotropic density variations found in liquids at medium distances are reflected in a damped oscillation reflecting the average molecular correlations.
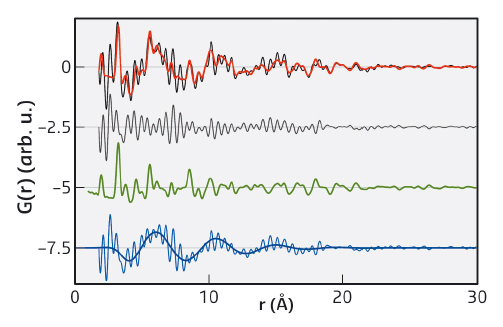
We discovered that the PDF of dry nanoparticles could be well explained by a simple crystal structure model. However, when the nanoparticles were dispersed in a range of solvents, an additional signal appeared. This takes the form of a damped oscillation like that seen in the bulk solvent (Figure 32). However, it is out of phase and cannot be subtracted as if it were a background contribution. After exhaustive experiments, we were able to show that this signal does not depend strongly on particle size, shape, nor the capping agent used. Supplementary measurement on metallic, polar and non-polar oxides showed that the formation of a solvation shell is apparently universal for colloidal nanoparticles.
 |
|
Fig. 32: Fit to the PDF of redispersed ZnO nanoparticles with citrate ligands in propanol. Experimental PDF of ZnO nanoparticles (black) and their fit (red), showing the overall difference of the fit (grey), the contribution of the nanoparticle (green), and the contribution and fit of the restructured solvent (blue), offset for clarity. The contribution of the restructured solvent (blue) is the difference-PDF of the experimental, background-corrected PDF (black) and the nanoparticles (green). |
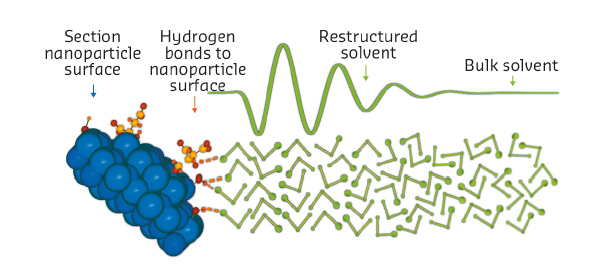
The signal is extremely weak, reflecting both the very low solubility of our nanoparticles (ca. 30-40 millimolar in ethanol), and the proportion of solvent in proximity to the particles. However, we were able to parameterise it using a simple model. The fitting shows that solvent restructuring extends as far as 2 nm into the bulk solvent. We also found that the signal shifts with alkyl chain length, so the alcohol molecules are likely to arrange perpendicular to the surface (Figure 33). Profound changes in the chemical properties of the solvent in this region are thus supported by our analysis.
 |
|
Fig. 33: Enhanced short-range order of solvent molecules at ZnO nanoparticle surfaces. The ethanol molecules (hydrogen atoms omitted) form hydrogen bonds with surface hydroxyl groups and citrate molecules. The surface coverage of these groups is reduced for clarity. The enhanced short-range order extends a few molecular layers into the bulk liquid. |
In summary, we have been able to detect the formation of a nanoscopic solvation shell around colloidal nanoparticles using high energy X-rays. Although the tiny solvent density changes reported here are at the very limit of detectability, when the ESRF-EBS lattice upgrade is complete, we should be able to detect changes in nanoparticle size, shape and chemistry, as well as the influence of the solvation shell in real time. This will open the door to a more complete understanding of the chemical and rheological properties of solvated colloidal nanoparticles.
Principal publication and authors
Universal solvent restructuring induced by colloidal nanoparticles, M. Zobel (a), R.B. Neder (a) and S.A.J. Kimber (b), Science 347, 292-294 (2015); doi: 10.1126/science.1261412.
(a) Department of Physics, Institute of Crystallography and Structural Physics, Friedrich-Alexander University Erlangen-Nuremberg (Germany)
(b) ESRF
References
[1] D.T. Bowron, S. Diaz-Moreno, J. Phys. Chem. B, 113, 11858 (2009).
[2] O.M. Magnussen et al., Phys. Rev. Lett., 74, 4444 (1995).
[3] D. Spagnoli et al., Langmuir, 27, 1821 (2001).



