- Home
- News
- Spotlight on Science
- The molecular details...
The molecular details of receptor-drug interactions in the treatment of asthma and heart conditions
14-11-2011
Researchers from the MRC Laboratory of Molecular Biology have unravelled how drugs such as sabutamol (Ventolin) interact with adrenergic receptors in the human body. These studies will aid future rational drug design to create more effective medicines and treatments of diseases such as asthma and heart disease.
Share
If ever you have been chased by lion, and lived to tell the tale, it is likely that you would report an elevated heart rate and rapid, deep breathing, even before you started to run. This is part of the ‘fight-or-flight’ response that allows you to deal with abnormally stressful situations and is mediated predominantly by the hormones adrenaline and noradrenaline that are released from the adrenal gland. These hormones are the core of the physiological responses observed under stress. For example, noradrenaline binds to a specific receptor on the surface of heart cells, the β1-adrenergic receptor (β1AR), and increases the rate of the heartbeat. Adrenaline binds to a very similar receptor in the lungs, the β2-adrenergic receptor (β2AR), where it acts to dilate the airways, thus allowing easier breathing. Both adrenaline and noradrenaline are known as agonists, as they activate their respective receptors, but how this happens at the molecular level was unclear until 2011, when a series of papers were published that showed for the first time how agonists interact with receptors.
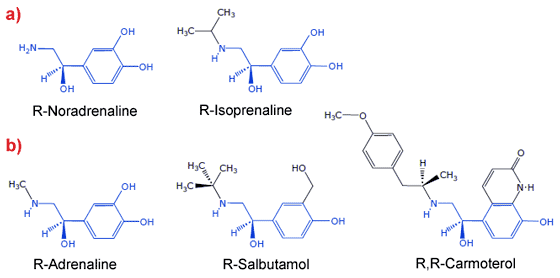
β1AR and β2AR are both G protein-coupled receptors (GPCRs), which are characterised by having seven transmembrane regions, with an extracellular N-terminus and intracellular C terminus. Although only 35-40 kDa in size, these receptors have proven very difficult to crystallise because they are extremely dynamic, undergoing a conformational change between an inactive R state and the active R* state even in the absence of ligands. This dynamism undoubtedly contributes to their instability in detergents, which are necessary to purify and crystallise any membrane protein. Unfortunately, the most useful detergents for crystallisation are often those that are most likely to denature the protein. We developed a thermostabilisation strategy that significantly improved the stability of receptors in short chain detergents, which allowed us to crystallise β1AR for the first time [1]. Subsequently, we crystallised the same receptor mutant bound to eight different ligands that either mimic the actions of noradrenaline, i.e. agonists, or block the binding of noradrenaline, i.e. antagonists. In fact, most of the compounds we used are either already prescribed as drugs for the treatment of heart conditions (antagonists of the β1AR), e.g. beta blockers, or are used to treat asthma (agonists of β2AR). Structures of some of the agonists are shown in Figure 1.
The high resolution structures of the thermostabilised β1AR mutant bound to the agonist isoprenaline highlights how little conformational change is required by the receptor to increase the probability of formation of the activated state. The initial binding of the agonist causes a contraction of the ligand binding pocket of the receptor by 1 Å and, combined with a change in rotamer conformation of a single serine residue, Ser215, is sufficient to induce the formation of the activated receptor. Once activation has occurred, a G protein can bind and be activated, which initiates a cascade of further reactions in the cytoplasm, ultimately leading to a physiological response by the cell. The small changes involved in receptor activation are exactly what would be expected for a sensitive system that can detect and amplify signals from the binding of a low molecular weight ligand.
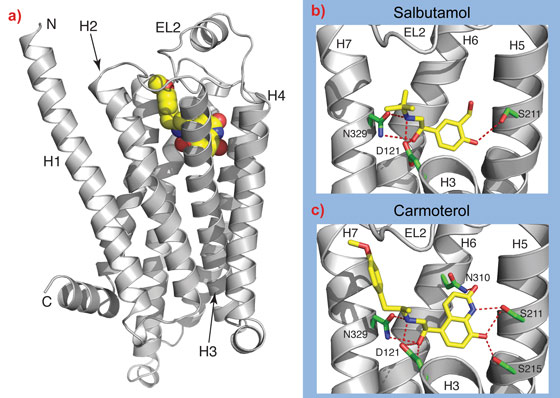
The biological insights from the structures are paralleled by the insights that may help in the design of new ligands that may be more effective treatments for asthma or heart conditions. For example, one of the structures we have determined of β1AR is bound to carmoterol, which was in phase III clinical trials as a one-shot per day treatment for asthma. Comparison of the carmoterol-bound β1AR structure with the structure with salbutamol bound, which is currently used for treating millions of asthmatics worldwide, shows that there are additional interactions that may decrease the rate of dissociation of the drug from the receptor and may therefore be instrumental in prolonging its biological activity (Figure 2).
GPCRs comprise an exciting group of receptors with regard to their huge potential for developing new therapeutics to a wide variety of diseases and afflictions, and determining their structures will accelerate the development of new therapeutics. The micro-focus beamline at ID23-2 is an essential tool in this process, as the crystals are invariably small and very radiation sensitive. The implementation of user-friendly scanning procedures for defining the best-diffracting part of a crystal or, in the case of lipidic cubic phase, to actually find the crystal in an opaque background, has been essential to improve throughput and to allow efficient screening of the hundreds of crystals that is typically required to collect a complete data set to high-resolution. None of the structures of GPCRs determined to date would have been possible without the technological advances and dedicated personnel that make these facilities available to structural biologists.
Principal publication and authors
The structural basis for agonist and partial agonist action on a β1-adrenergic receptor, T. Warne (a), R. Moukhametzianov (a), J.G. Baker (b), R. Nehmé (a), P.C. Edwards (a), A.G.W. Leslie (a), G.F.X. Schertler (a,c), C.G. Tate (a), Nature 469, 241-244 (2011).
(a) MRC Laboratory of Molecular Biology, Cambridge (UK)
(b) Institute of Cell Signalling, University of Nottingham (UK)
(c) Present address: Paul Scherrer Institut, Laboratory of Biomolecular Research, Villigen (Switzerland)
References
[1] T. Warne, M.J. Serrano-Vega, J.G. Baker, R. Moukhametzianov, P.C. Edwards, R. Henderson, A.G.W. Leslie, C.G. Tate & G.F.X. Schertler, Nature 454, 486-491 (2008).
Top image: Structure of the beta1-adrenergic receptor.