- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 1999
- Chemistry
- XAFS Measurements of Plutonium Hydrates
XAFS Measurements of Plutonium Hydrates
The first XAFS measurements of aqueous solutions containing Pu-242 were an important milestone for BM20 (ROBL CRG beamline). Such measurements contribute toward a better understanding of the behaviour of actinide elements in the environment. The goal of these first measurements was twofold. Firstly, we wanted to demonstrate that all requirements were met for the preparation and transportation of plutonium solutions from the home institution in Germany to the ESRF and for the XAFS measurement of these samples at BM20. This should encourage others to do similar experiments with radioactive samples. Secondly, the hydrate is the simplest chemical form of plutonium in aqueous solution. The knowledge of the structural parameters of the hydration sphere is important for the interpretation of EXAFS results on complicated aqueous plutonium complexes or sorbates where the water molecules are partly or fully replaced by other ligands. In general, the number of hydrating water molecules depends on such factors as ionic radius and charge and ion concentration.
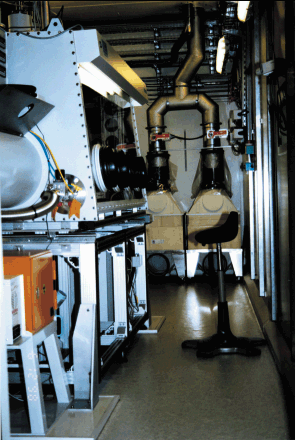
The Pu(VI) hydrate was prepared by dissolution of highly purified PuO2 (Pu-242) and electrochemical oxidation. Part of this solution was reduced to Pu(III) in an electrochemical cell. The Pu(III) and Pu(VI) hydrates were in perchloric and nitric media (1 M acidic solutions), respectively. The final concentration of Pu was 0.05 mol/L. The Pu oxidation states were confirmed by UV/Vis spectroscopy. About 4.7 mL of solution (7.5 MBq) was filled and sealed in polyethylene cuvettes. The samples were shipped by special transportation from Forschungszentrum Rossendorf in Dresden, Germany to the ESRF. Forty-eight hours after their preparation, the Pu solutions were positioned for the XAFS measurements inside the glove box of the radiochemistry hutch at ROBL (see photograph). ROBL is the only experimental station at the ESRF and one of few stations worldwide that is dedicated to XAFS studies on solid and liquid radioactive samples with a maximal activity of 185 MBq. According to the safety requirements for a radiochemistry laboratory, the glove box is complemented by a safety system with separate ventilation, air filtering and air monitoring systems [1]. The Pu LIII-edge (18057 eV) XANES and EXAFS spectra were measured at room temperature in transmission mode using the Si(111) double-crystal monochromator and two Pt coated mirrors for rejection of higher harmonics.
As one can see from Figure 35, the LIII absorption edge of Pu(VI) is shifted by 4 eV toward higher energy as compared to that of Pu(III). The distinct XANES features of these two Pu hydrates have been modelled by a relativistic multiple-scattering calculation [2]. The different electronic and molecular structures of Pu(III) and Pu(VI) hydrates are also reflected in the EXAFS shown in Figure 36. The coordination sphere of Pu(III) hydrate can be written as Pu(H2O)83+ with an average Pu-O bond distance of 2.48 Å. The Fourier transform corresponding to the EXAFS of Pu(VI) hydrate shows two coordination shells. The Pu(VI) forms a plutonyl ion PuO2(H2O)4-52+. The axial and equatorial Pu-O bond distances are 1.74 and 2.42 Å, respectively. The structural parameters of Pu(VI) hydrate are nearly identical to those of U(VI) and Np(VI) hydrates, which were measured recently at BM20 [3]. Studies of other Pu oxidation states are in preparation.
References
[1] W. Matz et al., J. Synchrotron Rad., 6, 1076 (1999).
[2] A.L. Ankudinov et al., Phys. Rev. B., 57, 7518 (1998).
[3] T. Reich et al., Radiochim. Acta (accepted for publication).
Authors
T. Reich, G. Geipel, H. Funke, C. Hennig, A. Rossberg, G. Bernhard.
Forschungszentrum Rossendorf, Institute of Radiochemistry, Dresden (Germany)