- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2000
- Chemistry
- Structure and Mobility of Cu(I) Cations in Cu(I)-Y Zeolite: a Combined X-ray Powder Diffraction and EXAFS Study on the Effect of Interaction with CO
Structure and Mobility of Cu(I) Cations in Cu(I)-Y Zeolite: a Combined X-ray Powder Diffraction and EXAFS Study on the Effect of Interaction with CO
Zeolites are nanoporous crystalline aluminosilicates constituted by corner-sharing [TO4] tetrahedra, where T represents a silicon or an aluminum atom, with chemical composition described by the general formula Mn+x/n[(AlO2)x(SiO2)y]x. The introduction of a trivalent Al(III) atom in a [TO4] unit (substituting the tetravalent Si(IV) atom), induces a net negative charge into the zeolitic framework (x-) which must be compensated by the presence of charge-balancing extra-framework cations (Mn+x/n). Such cations can act as active centres in acid-catalysed reactions (M+ = H+) or in redox reactions (Mn+ = a transition metal ion).
Cu-exchanged zeolites have recently attracted great interest as catalysts for the direct conversion of NO into N2 and O2 and for the selective reduction of NO with ammonia and hydrocarbons. A thorough characterisation of these catalysts needs (i) the structural localisation of the active centres, (ii) the study of the reactivity of Cu(I) ions by the interaction with probe molecules like CO or NO, and (iii) the monitoring of the complete Cu(I) ![]() Cu(II) reduction/oxidation cycle. Point (iii) has been discussed in [1,2] using EPR, IR, UV-Vis, XPS and XANES experiments.
Cu(II) reduction/oxidation cycle. Point (iii) has been discussed in [1,2] using EPR, IR, UV-Vis, XPS and XANES experiments.
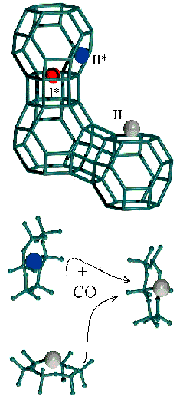 |
Fig. 20: Representation of the cation location for Cu(I)-Y in vacuo (top) and their migration upon adsorption of CO (bottom) as deduced from the Rietveld refinement of the XRPD data collected in situ at 80 K at BM16.
|
The siting of copper ions in Cu(I)-Y zeolite, prepared by gas-phase exchange of H-Y with CuCl, has been investigated employing X-ray powder diffraction (XRPD) and EXAFS data collected in situ at the beamlines BM16 and BM8 (GILDA), respectively. The Rietveld refinement of the XRPD data of the zeolite in vacuo shows that 23.4(2) cuprous ions are located at site I*, 6.1(3) at site II, and 11.5(3) at site II* (see top part of Figure 20). From the XRPD study we were able to infer the occupancy-weighted EXAFS signal expected for the three families of Cu(I) sites, exhibiting 3 equivalent oxygen atoms at 2.00 Å, 2.03 Å and 2.21 Å, respectively, (see the first three spectra from top in Figure 21). The results obtained from the one-shell fit of the EXAFS signal obtained by adding the contribution from the three families (bottom spectrum in Figure 21) were in full agreement with the one-shell fit of the experimental EXAFS signal.
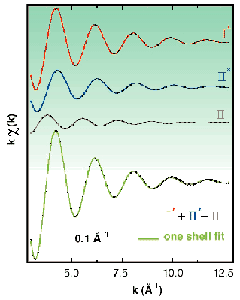 |
Fig. 21: Simulated EXAFS spectra of the contribution to the overall signal of Cu(I) located in (from top to bottom) site I*, II* and II. In the simulation, R and N values have been obtained from the XRPD data. The sum of the three simulated spectra has than been fitted using a single shell of oxygen scatterers. The results coming from this simulation are in full agreement with the one-shell fit performed on the experimental EXAFS spectrum collected at the BM8 (GILDA) beamline.
|
Coming to point (ii), the results obtained from synchrotron radiation techniques are supported by parallel microcalorimetry and FTIR studies of adsorbed CO. From the combined use of the three techniques we learn that CO is adsorbed on the Cu(I)-zeolites at ambient temperature with the formation of Cu(I)(CO) adducts, which evolve into Cu(I)(CO)2 upon increasing the CO pressure (PCO) [2,3]. At 80 K, and low PCO, FTIR indicates that two types of monocarbonyl species are observed in Cu(I)-Y, corresponding to CO adsorbed on copper ions located at sites II and II*. On increasing PCO, and subsequent formation of polycarbonylic species, cations at site II* move to the more-exposed position II and only a kind of Cu(I)(CO)3 adduct is observed by FTIR, in full agreement with the XRPD data (bottom part of Figure 20).
References
[1] G. Turnes Palomino, P. Fisicaro, S. Bordiga, A. Zecchina, E. Giamello and C. Lamberti, J. Phys. Chem. B, 104, 4064-4073 (2000).
[2] V. Bolis, S. Maggiorini, L. Meda, F. D'Acapito, G. Turnes Palomino, S. Bordiga and C. Lamberti, J. Chem. Phys. 113, 9248-9261 (2000).
[3] C. Lamberti, G. Turnes Palomino, S. Bordiga, G. Berlier, F. D'Acapito and A. Zecchina, Angew. Chem. Int. Ed., 39, 2138-2141 (2000).
Principal Publication and Authors
G. Turnes Palomino (a,b), S. Bordiga (a), A. Zecchina (a), G.L. Marra (c) and C. Lamberti (a,d), J. Phys. Chem. B, 104, 8641-8651 (2000).
(a) University of Turin (Italy)
(b) UIB (Spain)
(c) EniChem S. p. A. (Italy)
(d) INFM Sezione di Torino (Italy)



