- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2000
- Surfaces and Interfaces
- Following Local Adsorption Sites through a Surface Chemical Reaction: CH3SH on Cu(111)
Following Local Adsorption Sites through a Surface Chemical Reaction: CH3SH on Cu(111)
Practical surface chemical reactions, such as those in heterogeneous catalysis, involve several coadsorbed molecular moieties, including reaction intermediates, but little progress has been made so far in determining their local adsorption geometries. At ID32 we have achieved this goal by combining the standard method of normal-incidence X-ray standing waves (NIXSW) [1] with detection of the photoabsorption cross-section at different sites through the intensities of the chemically-shifted photoemission peaks.
The interaction of alkane thiols with surfaces is of interest both from the point of view of desulphurisation catalysts and catalyst poisoning, and because these species form self-assembled monolayers which are of potential interest in molecular electronics. Methanethiol, CH3SH, is the simplest such species, and its interaction with Cu(111) has recently been characterised by high-resolution S 2p photoelectron spectroscopy [2]. Four distinct S-containing surface species result, identified as adsorbed intact methanethiol (temperature < 150 K), two distinct states both attributed to methyl thiolate (CH3S-) adsorbed in different geometries, and atomic sulphur, to which all species transform at high temperature. We label the two thiolate species low and high temperature (LT and HT); both thiolates are present at low temperature, but the LT thiolate transforms to the HT thiolate as the temperature is raised.
In XSW an X-ray Bragg reflection is established in the substrate and the interference of the incident and diffracted beams produces a standing wavefield, the phase of which shifts with varying photon energy; this variation of X-ray absorption at particular atoms is characteristic of their location relative to the scatterer planes. Normal incidence to the scatterer planes reduces dependence on substrate crystal perfection and allows standard metal single crystals to be used, while the use of (111) and (![]() 11) Bragg reflections provides absorber-substrate spacings sufficient to triangulate the absorber atom locations. The X-ray absorption at S atoms in the adsorbates was determined from the S 1s photoemission spectra, which could be separated into the individual chemically-shifted components, providing independent XSW absorption profiles of the coadsorbed species. Figure 50 shows a subset of such spectra recorded at six different photon energies with the surface at 140 K for the (111) reflection; the relative intensities of the different chemically-shifted S 1s components varies significantly, demonstrating that different adsorption sites are associated with each state. The locations of these six photon energies relative to the substrate XSW absorption wave profile are shown in the right-hand panel of Figure 50.
11) Bragg reflections provides absorber-substrate spacings sufficient to triangulate the absorber atom locations. The X-ray absorption at S atoms in the adsorbates was determined from the S 1s photoemission spectra, which could be separated into the individual chemically-shifted components, providing independent XSW absorption profiles of the coadsorbed species. Figure 50 shows a subset of such spectra recorded at six different photon energies with the surface at 140 K for the (111) reflection; the relative intensities of the different chemically-shifted S 1s components varies significantly, demonstrating that different adsorption sites are associated with each state. The locations of these six photon energies relative to the substrate XSW absorption wave profile are shown in the right-hand panel of Figure 50.
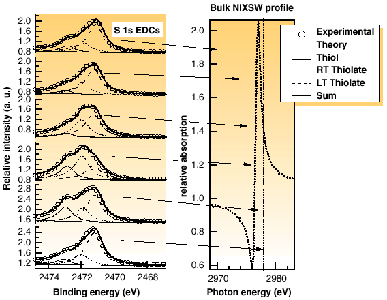 |
Fig. 50: Experimental S 1s photoelectron energy spectra (left) from Cu(111) exposed to a saturation dose of CH 3SH at 140 K, recorded at different photon energies through the (111) NIXSW condition. Also shown (right) is the bulk (Cu) absorption profile through this region showing the relative locations of these four photon energies (a) to (d).
|
By fitting the S chemical-shift XSW absorption profiles from a far more complete set of data, the local adsorption structure of each species could be determined. The intact thiol at low temperature is bonded to the Cu(111) surface through its S atom which is directly atop an outermost layer Cu atom with a Cu-S bond length of 2.38 Å. The LT thiolate species are found to occupy either mixed hollow or bridging sites, while the RT thiolate induces a reconstruction of the outermost Cu layer to lower density and occupies modified hollow sites. The atomic S occupies 'fcc' hollow sites, directly above third layer Cu atoms. Figure 51 shows a schematic of these local geometries on the unreconstructed surface.
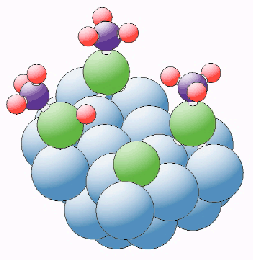 |
Fig. 51: Schematic diagram showing the local adsorption geometries at low temperature of the coadsorbed intact thiol (left) and LT thiolate on Cu(111); also shown is the HT atomic S species.
|
References
[1] D.P. Woodruff, Prog. Surf. Sci. 57, 1-60 (1998).
[2] M.S. Kariapper, G.F. Grom, G.J. Jackson, C.F. McConville and D.P. Woodruff, J. Phys. Condens. Matter, 10, 8661-8670 (1998).
Principal Publication and Authors
G.J. Jackson (a), D.P. Woodruff (a), R.G. Jones (b), N.K. Singh (b,c), A.S.Y. Chan (b), B.C.C. Cowie (d,e) and V. Formoso (e), Phys. Rev. Lett. 84, 119-122 (2000).
(a) University of Warwick (UK)
(b) University of Nottingham (UK)
(c) University of New South Wales, Kensington (Australia)
(d) CLRC Daresbury Laboratory, Warrington (UK)
(e) ESRF



