- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2004
- X-ray Imaging
- First Direct Observation of Brine Droplets and Solid Inclusions in Accreted Ice from the Sub-glacial Lake Vostok (Antarctica)
First Direct Observation of Brine Droplets and Solid Inclusions in Accreted Ice from the Sub-glacial Lake Vostok (Antarctica)
The largest sub-glacial lake (14,000 km2) discovered by a radio-echo survey under the Antarctic ice cap is covered by 3,750 m of ice and underlies the Russian station of Vostok where a 3620 m deep ice core has been recovered. Ice coring has been stopped 130 m above the lake surface, in ice formed by the refreezing of lake water at the bottom of the glacier. Although made of large ice crystals of very high crystalline quality, accretion ice contains many visible inclusions and large amounts of soluble species (sulphate salts and NaCl) [1]. The origin of these impurities is puzzling and information on their composition and location is essential for the understanding of the lake's environment and mechanisms driving ice formation and aging process.
We present here data obtained by X-ray fluorescence at beamline ID21. The microprobe was set at 4.5 keV for 2D mapping of elements lighter than Ca. A specific experiment was carried out for sulphur species, tuning the excitation energy to around 2.5 keV. The shift in energy produced a clear distinction between sulphur present as sulphide (SII-, 2.470 keV), sulphite (SIV, 2.478 keV) and sulphate (SVI, 2.482 keV) [2,3]. The cryosystem allowed the temperature of the sample holder to be kept stable and close to -140°C. The cell containing the ice sample was tightly closed by a film of "ultralene"®, making it possible to study the upper 50 µm of samples for several hours under high vacuum (2 x 10-5 kPa) conditions without any visual change of the ice surface. In this experiment, the plastic film was mechanically fastened to the top of the cell to avoid chlorine contamination from glue.
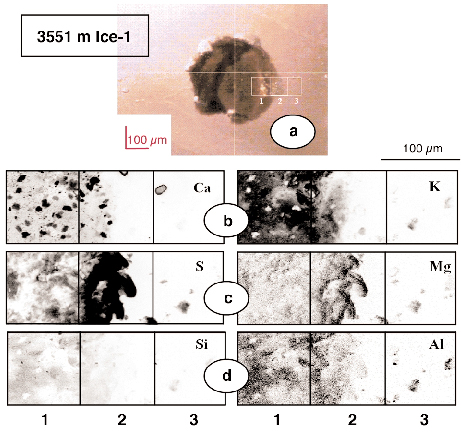
Four accretion ice samples containing visible inclusions and one grain boundary have been studied. A very large inclusion was carefully investigated (Figure 133).This inclusion was a very large aggregate of various particles including sulphur compounds, alumino-silicates and other silicate species, and relatively large carbonates. Several smaller dark aggregates showing rather similar composition were found inside ice crystals, while the grain boundary structure contained only very few particles per µm. One aggregate was studied for sulphur speciation, showing that reduced sulphur species were present along with sulphate salts.
 |
|
Fig. 133: Elemental maps of three contiguous areas of a very large solid inclusion (600 µm) at 3551 m. Beam energy was focused at 4.5 keV. Probe size was 1 x 1 µm2. The highest concentration of the total area represented in elemental mapping is automatically scaled to black. |
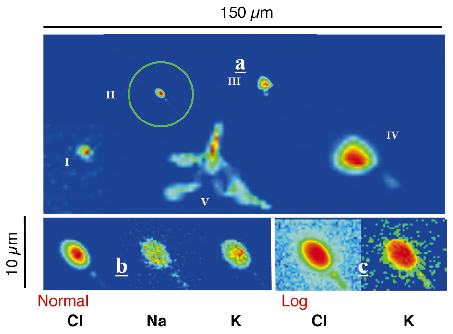
Chlorine was almost exclusively detected in colourless micrometer-sized structures (Figure 134), which are very likely to be brine micro-pockets entrapped in the ice lattice. A rough estimate of their salinity leads to a value (1.5%) significantly higher than the maximum salinity calculated for the main lake[1]. At the temperature prevailing in accreted ice (-3°C), brine should remain in a liquid state. During drilling, the outer pressure decreases from 30 to 0.1 MPa and ice cores are cooled to about -50°C. The relaxation and the freezing of water droplets can initiate ice cracks along crystallographic planes, which could explain the shape and orientation of bubble extensions clearly visible in Figure 134c. The freezing of brine droplets could also lead to concentration gradients as observed in brine bubbles (Figure 134b), solute exclusion occurring at the ice water interface during the freezing process.
 |
|
Fig. 134: Brine inclusions at 3551 m. Five colourless oblong objects were observed over an area of 200 x 200 µm2, with apparent diameter varying from ~ 3 to ~ 15 µm. Object V exploded during the scan. Probe size ranged from 0.3 x 0.5 µm2 (II and III) to 1 x 1 µm2 (I, IV and V). The intensity scale extends from dark blue to red. |
This work is, to our knowledge, the first direct observation of well-preserved liquid microstructures along with other solid and complex objects in ice. Accretion ice from lake Vostok contains solid particles initially boosted by saline water in frazil slush. Single crystals that growing once the ice is accreted may keep large solid aggregates as well as haline droplets inside the ice lattice. The structure and composition of solid inclusions linked to the high salinity of brine droplets provide new arguments for the occurrence, under lake Vostok, of saline water pulses through a deeper sulphur rich sedimentary reservoir.
References
[1] M. De Angelis, J.R. Petit, J. Savarino, R. Souchez , and M.H. Thiemens, Earth Planet. Sci. Lett. 222, 751-765 (2004).
[2] M. Stöhr, NEXAFS Spectroscopy, Berlin: Springer-Verlag (1992).
[3] J. Prietzel, J. Thieme, U. Neuhaeusler, J. Susini and I. Kogel-Knabner, European J. Soil Sci. 54, 423433 (2003).
Principal Publication and Authors
M. de Angelis (a), M.-C. Morel-Fourcade (a), J.-M. Barnola (a), J. Susini (b) and P. Duval.
(a) LGGE/CNRS Saint Martin d'Hères (France)
(b) ESRF



