- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2004
- Soft Condensed Matter
- Structural Evolution of Regenerated Silk Fibroin: Combined
Structural Evolution of Regenerated Silk Fibroin: Combined
During the spinning process of spiders or silk worms, solvated random-coil fibroin protein is transformed into a dry, semicrystalline silk thread [1]. It is often assumed that a silk I structure is initially formed and subsequently transformed into the b-sheet type silk II structure [2] under the action of shearing forces in the tapered end of the spinneret. An experimental proof is, however, lacking and in vivo experiments on dragline silk production showed only the presence of the silk II structure.
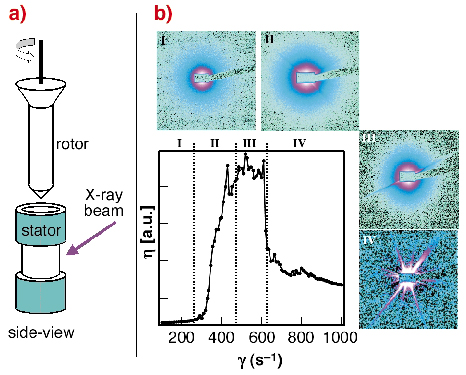
In order to simulate the shearing forces occurring during the spinning process we have studied the solidification process of regenerated Bombyx mori silk using the Couette geometry on the rheometer (Figure 51a) at beamline ID02 by an in situ SAXS/WAXS experiments.
 |
|
Fig. 51: a) Schematic design of the Couette cell geometry used at beamline ID02; b) change of the viscosity ( |
The change of viscosity with shearing rate can be roughly separated into four zones. A non-Newtonian behaviour was observed starting in zone II. Analysis of the scattering data suggests a transition of the random-coil fibroin into a more compact, folded fibroin, presumably due to an onset of hydrogen-bonding. Aggregation of fibroin starts in zone III as evidenced by streaks. Due to the formation of large fibroin aggregates in zone IV the viscosity is decreasing. WAXS data show that the precipitated fibroin is amorphous. Complementary ATR-FTIR data indicate the presence of b-sheet conformation.
 |
|
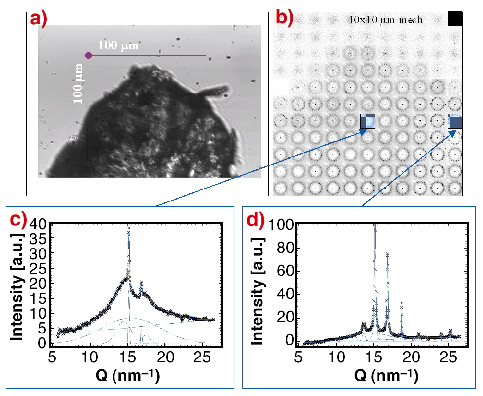
Fig. 52: a) Optical image of precipitated and partially dried sample. The 100 x 100 µm zone was mesh-scanned by a 5 µm X-ray beam; b) composite picture of the mesh-scan: the individual "pixels" correspond to SAXS/WAXS patterns recorded with a MAR CCD; c) azimuthally-averaged WAXS-pattern from the edge showing a mixture of broad reflections (silk II) and narrow reflections of a second phase, which probably corresponds to the silk I structure. The broad reflections of the silk II phase are identical to the reflections from a Bombyx mori fibre; d) practically pure second phase (silk I) reflections. |
The formation of the silk I/silk II structures during the drying process of a piece of precipitated fibroin (Figure 52a) was investigated by micro-SAXS/WAXS experiments at beamline ID13. Figure 52b shows a composite picture obtained by a 10 x 10 µm mesh-scan with a 5 mm beam.
The data (Figure 52c and 52d) agree to the presence of two phases: (i) a silk II phase with rather broad reflections and (ii) a second phase with narrow reflections corresponding probably to a hydrated silk I structure. As the two phases coexist in different ratios across the scanned zone one can assume that the drying process proceeds through an intermediate hydrated silk I phase, which is subsequently transformed into the unhydrated silk II structure.
References
[1] F. Vollrath and D.P. Knight, Nature 410, 541-548 (2001).
[2] R.D.B. Fraser and T.P. MacRae, Conformations of Fibrous Proteins, New York: Academic Press. (1973).
Principal Publications and Authors
M. Rössle (a), P. Panine (b), V.S. Urban (c) and C. Riekel (b), Biopolymers 74(4), 316-327 (2004).
(a) EMBL Outstation Hamburg (Germany)
(b) ESRF
(c) Oak Ridge National Laboratory (USA)



