- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2005
- Structural Biology
- Crystal Structure of Maltooligosyltrehalose Trehalohydrolase from D. radiodurans
Crystal Structure of Maltooligosyltrehalose Trehalohydrolase from D. radiodurans
Deinococcus radiodurans is a bacterium that displays an extraordinary resistance to a wide-range of DNA-damaging agents, such as ionising radiation and desiccation [1]. Since there are no terrestrial environments that generate high doses of radiation, it has been proposed that the radioresistance of D. radiodurans has arisen as a by-product of selection for desiccation resistance.
The removal of water from a cell is a severe, often lethal stress. During desiccation, cell metabolism is completely arrested and subsequently needs to be fully recovered during rehydration and extensive damage is caused to DNA, proteins and lipid membranes. Certain sugars have been shown to contribute to the stability of proteins, membranes and whole cells during prolonged dehydration. In particular, trehalose appears to be the most effective stabiliser of dried proteins and membranes. Trehalose is a non-reducing ![]() -1,1-linked disaccharide (
-1,1-linked disaccharide (![]() -D-glucopyranosyl-1,1-
-D-glucopyranosyl-1,1-![]() -D-glucopyranose) that accumulates in organisms under stress conditions. Evidence suggests that trehalose protects membranes and proteins by serving as a water substitute [2].
-D-glucopyranose) that accumulates in organisms under stress conditions. Evidence suggests that trehalose protects membranes and proteins by serving as a water substitute [2].
D. radiodurans uses an unusual pathway for trehalose biosynthesis. Two enzymes, maltooligosyltrehalose synthase (MTSase) and maltooligosyltrehalose trehalohydrolase (MTHase), catalyse the breakdown of maltooligosaccharides or starch into trehalose. MTHase is a member of the ![]() -amylase family of (ß/
-amylase family of (ß/![]() )8 barrel glycosidases, which comprises enzymes with a wide variety of known activities. We have determined the high resolution (1.1 Å) crystal structure of D. radiodurans MTHase (DrMTHase) using the single-wavelength anomalous dispersion (SAD) method and data collected on beamlines ID29 and ID14-2. Crystal structures were obtained for both apo- and disaccharide-bound (maltose and trehalose) forms of DrMTHase.
)8 barrel glycosidases, which comprises enzymes with a wide variety of known activities. We have determined the high resolution (1.1 Å) crystal structure of D. radiodurans MTHase (DrMTHase) using the single-wavelength anomalous dispersion (SAD) method and data collected on beamlines ID29 and ID14-2. Crystal structures were obtained for both apo- and disaccharide-bound (maltose and trehalose) forms of DrMTHase.
 |
|
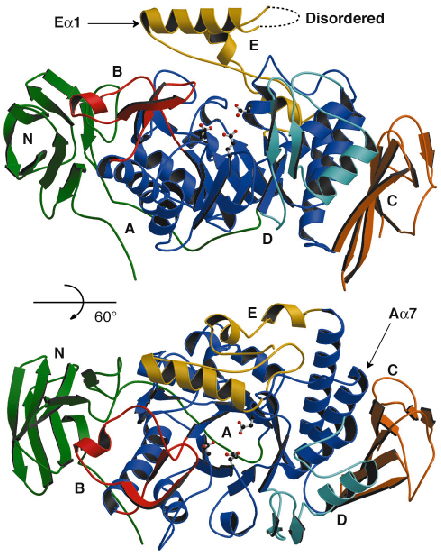
Fig. 86: Ribbon representation of the crystal structure of DrMTHase. Each domain is represented in a different colour and the catalytic residues are illustrated in ball-and-stick representation. |
DrMTHase is a monomer and displays three major domains (Figure 86): an N-terminal domain (domain N) that consists of an eight-stranded immunoglobulin-like Greek key fold, a central catalytic (ß/![]() )8 barrel domain (domain A) and a C-terminal domain (domain C) that consists of a six-stranded
)8 barrel domain (domain A) and a C-terminal domain (domain C) that consists of a six-stranded ![]() -crystallin-type fold. Three additional subdomains (domains B, D and E) protrude from the central domain A (Figure 86). The structures of DrMTHase in complex with either maltose (an
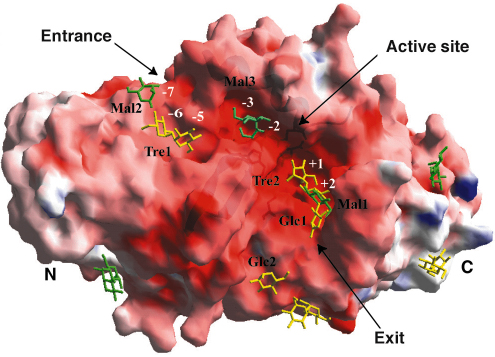
-crystallin-type fold. Three additional subdomains (domains B, D and E) protrude from the central domain A (Figure 86). The structures of DrMTHase in complex with either maltose (an ![]() -1,4-linked disaccharide, which mimics a short portion of the substrate) or trehalose (the product of the reaction) reveal that all three inserted subdomains B, D and E are crucial for substrate binding. As shown in Figure 87, two channels are formed at the surface of DrMTHase to bind long sugar chains, one leading down to the active site and another guiding the sugars out again. Unlike other members of the glycosidase family, DrMTHase undergoes a significant conformational change upon substrate (maltose) binding, in order to guide the sugar chain into the active site. Subdomains B and E trap the substrate in the active site and most likely maintain the sugar chain in a strained conformation in order to lower the energy barrier of hydrolysis.
-1,4-linked disaccharide, which mimics a short portion of the substrate) or trehalose (the product of the reaction) reveal that all three inserted subdomains B, D and E are crucial for substrate binding. As shown in Figure 87, two channels are formed at the surface of DrMTHase to bind long sugar chains, one leading down to the active site and another guiding the sugars out again. Unlike other members of the glycosidase family, DrMTHase undergoes a significant conformational change upon substrate (maltose) binding, in order to guide the sugar chain into the active site. Subdomains B and E trap the substrate in the active site and most likely maintain the sugar chain in a strained conformation in order to lower the energy barrier of hydrolysis.
 |
|
Fig. 87: Illustration of the electrostatic surface potential of DrMTHase in which red and blue represent negative and positive potentials respectively. Bound sugars are represented in green (maltose) and yellow (trehalose). |
The presence of trehalose in the active site of the DrMTHase-trehalose complex has allowed the identification of the residues involved in the selective binding of the ![]() -1,1-linked sugar, trehalose, as opposed to other maltooligosaccharides. The sugar units are specifically recognised by an extensive hydrogen bonding network, in which many water molecules are involved and are further stabilised by hydrophobic stacking interactions in between two protein aromatic side chains. Importantly, the His332 N
-1,1-linked sugar, trehalose, as opposed to other maltooligosaccharides. The sugar units are specifically recognised by an extensive hydrogen bonding network, in which many water molecules are involved and are further stabilised by hydrophobic stacking interactions in between two protein aromatic side chains. Importantly, the His332 N![]() 2 atom hydrogen-bonds directly to the O6 atoms of the two sugar units of the bound trehalose molecule. This interaction is trehalose specific, since the presence of both O6 atoms at hydrogen-bonding distance from His332 N
2 atom hydrogen-bonds directly to the O6 atoms of the two sugar units of the bound trehalose molecule. This interaction is trehalose specific, since the presence of both O6 atoms at hydrogen-bonding distance from His332 N![]() 2 could only occur with a
2 could only occur with a ![]() -1,1-linked disaccharide.
-1,1-linked disaccharide.
This work has revealed the mode of substrate recognition of DrMTHase, which is most likely shared by other members of the subfamily of MTHase enzymes, since most of the critical residues are conserved. The presence of this unusual trehalose synthesis pathway in the extreme radiation and desiccation-resistant bacterium D. radiodurans suggests that this pathway may be essential to its survival, especially as the ‘classical’ pathway involving trehalose-phosphate synthase is missing. The rapid breakdown of the most widely available source of maltodextrins in nature, i.e. soluble starch, would be an efficient mechanism to rapidly produce trehalose in response to environmental stress.
References
[1] V. Mattimore, & J. R. Battista. J. Bacteriol. 178, 633-637 (1996).
[2] M. Potts. Trends Microbiol. 9, 553-559 (2001).
Principal Publication and Authors
J. Timmins (a), H.-K. Leiros (a), G. Leonard (a), I. Leiros (a) and S. McSweeney (a). J. Mol. Biol. 347 (5), 949-963 (2005).
(a) ESRF



