- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2005
- X-ray Absorption and Magnetic Scattering
- XAFS and Vibrational Characterisation of the Local Structure of Framework Cu(II) in HKUST-1 Metallorganic Framework
XAFS and Vibrational Characterisation of the Local Structure of Framework Cu(II) in HKUST-1 Metallorganic Framework
Metal Organic Frameworks (MOFs, also known as “Coordination Polymers”) are crystalline nanoporous materials comprised of small metal-containing clusters connected three-dimensionally by poly-functional organic ligands. The ligands act as spacers, creating an open porous three-dimensional structure, with very high pore volume and surface area. This hybrid architecture opens the possibility to design and synthesise a great variety of new porous materials, which are in principle able to display novel functionalities that are potentially exploitable for a number of applications such as gas storage, non linear optics, ion-exchange and catalysis.
In the past MOFs were disavantaged by the lack of control of polymer dimensionality and poor framework stability. More recently, new MOFs have been synthesised, overcoming the above limitations in that they exhibit permanent porosity also after evacuation and template removal.
 |
|
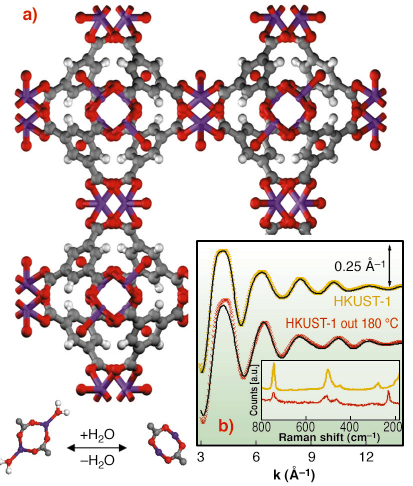
Fig. 111: (a): ball and stick representation of HKUST-1 MOF: O (red), C (gray) H (white) Cu (purple). The lower part represents the dehydration process of the [Cu2C4O8] cage (top view); (b): EXAFS k |
This work deals with HKUST-1 [1], a copper based fcc-MOF characterised by a 3D system of large square-shaped pores (9 Å by 9 Å) (Figure 111a). In HKUST-1 Cu2+ ions form dimers, where each copper atom is coordinated by four oxygens, coming from the benzene-1,3,5 tricarboxilate linkers ([Cu2C4O8] cage) and one water molecule (bottom part of Figure 111a). We have shown that, upon an appropriate activation procedure, the water molecule can be removed from the first coordination shell of Cu2+ without loss of crystallinity. EXAFS (Figure 111b), collected on BM29, indicates that H2O removal resulted in a shrinking of the [Cu2C4O8] cage due to water desorption, with the copper atoms that sink in the plane of carbossilic oxygens due to a shortening of the first shell Cu-O bond and to a reduction of the Cu-O-C angle, as depicted in Figure 111a (bottom part). This structural rearrangement has consequences on the vibrational modes of the framework as evidenced by a Raman study (inset of Figure 111b) and on the XANES spectrum Figure 112.
 |
|
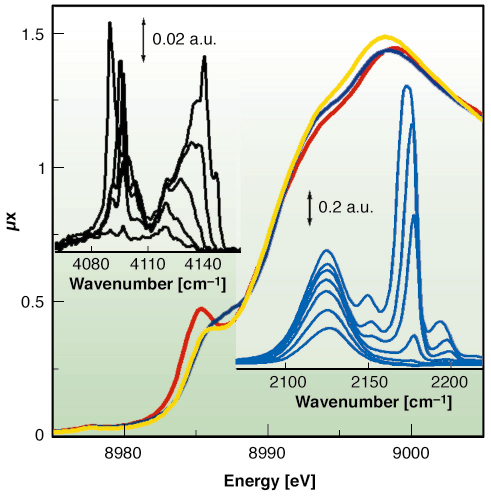
Fig. 112: XANES spectra of HKUST-1 sample collected at 77 K before (yellow) and after (red) dehydration and after CO adsorption (blue). Top and bottom insets report the IR spectra collected at increasing coverage of H2 (at 20 K) and of CO (at 77 K), respectively. |
We have proven that Cu2+ species in the dehydrated material are able to coordinate CO at 77 K, as evidenced by XANES (Figure 112) and IR data (bottom inset). This is the first direct evidence of the extensive formation of Cu2+···CO adducts inside a porous hosting matrix, compared to the Cu+···CO complexes largely reported in the literature [2]. As already reported for MOF-5 [3], by lowering the temperature down to 20 K also Cu2+···H2 adducts have been observed with IR, as testified by the top inset of Figure 112.
References
[1] S.S.Y. Chui, S.M.F. Lo, J.P.H. Charmant, A.G. Orpen, I. D. Williams, Science 283 1148-1150 (1999).
[2] C. Lamberti, G. Turnes Palomino, S. Bordiga, G. Berlier, F. D’Acapito and A. Zecchina, Angew. Chem. Int. Ed., 39, 2138-2141 (2000).
[3] S. Bordiga, J.G. Vitillo, G. Ricchiardi, L. Regli, D. Cocina, A. Zecchina, B. Arstad, M. Bjørgen, J. Hafizovic, K.P. Lillerud, J. Phys. Chem. B, 109, 18237-18242 (2005).
Principal publication and Authors
C. Prestipino (a), L. Regli (a), J.G. Vitillo (a), F. Bonino (a), A. Damin (a), C. Lamberti (a), A. Zecchina (a), P.L. Solari (b), K.O. Kongshaug (c), S. Bordiga (a), Chem. Mater., 18, (2006), in press.
(a) NIS centre of excellence University of Turin, and INSTM (Italy)
(b) ESRF (France)
(c) Department of Chemistry, University of Oslo (Norway)



