- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2006
- High Resolution and Resonance Scattering
- Unequivocal detection of the 3d4 high-spin system by resonant inelastic X-ray scattering
Unequivocal detection of the 3d4 high-spin system by resonant inelastic X-ray scattering
The electronic structure of 3d transition metals is subject to intense studies. Chemists classify transition metal compounds by means of their oxidation states. The most common oxidation states for Fe are II and III. In a purely ionic picture this corresponds to 3d6 and 3d5 valence shell configurations, respectively. A higher oxidation state means that the half-filled 3d shell opens up, which significantly changes the Fe chemistry. High oxidation states of iron play an important role in biological systems. Oxoiron(IV) species are found in heme enzymes and Fe(IV) intermediates are important parts of the catalytic cycles of methane monooxygenase, an enzyme capable of oxidising the C-H bond in methane as well as other alkanes. In solid state chemistry high oxidation states of iron are less common. The most well known examples for a material with formal oxidation state Fe(IV) are the perovskite FeSrO3-x and related materials with similar composition [1]. They play an important role in catalysis, in gas-sensors, electrochemistry and in solid state physics.
One strongly debated scientific case is Fe in a zeolite framework system (Fe-ZSM-5). The involvement of Fe(IV) species was invoked to explain the high activity and selectivity of Fe-ZSM-5 catalysts in the oxidation of benzene to phenol [2]. The reaction of N2O with Fe(II) sites in ZSM-5, generated by autoreduction at high temperatures, creates a highly reactive surface oxygen species, which selectively oxidises benzene at moderate temperatures. Experimental proof of the existence of Fe(IV) in Fe-ZSM-5 has not yet been given because one has to rely on a phenomenological interpretation of the line shifts in Mössbauer and XANES spectra, which is often ambiguous for heterogeneous and strongly covalent systems. Hence, the identification of the oxidation state of iron in Fe-ZSM-5 requires an alternative solution.
Spin-selective absorption spectroscopy takes advantage of the strong (3p,3d) exchange interaction in the final states of 1s3p resonant inelastic X-ray scattering (RIXS) spectroscopy [3]. This interaction splits the Kß emission lines into the Kß1,3 (spin-down) and Kß’(spin-up) features. Using an emission spectrometer with an instrumental energy bandwidth of ~1 eV, it is possible to distinguish between final states that correspond to a spin-up or spin-down orientation of the unpaired 3p electron with respect to the valence electrons. This translates directly into the spin orientation of the photoexcited 1s electron thus allowing detection of the spin-up and spin-down density of unoccupied electronic states, respectively.
 |
|
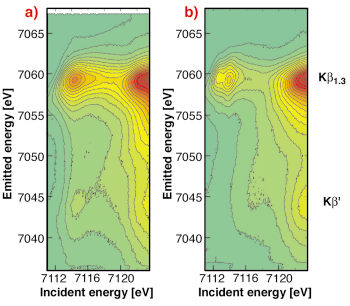
Fig. 8: 1s3p RIXS planes of a) FeSrO2.89 and b) Fe2O3. The spectra were taken at ID26. |
A high-spin 3d5 (Fe (III)) configuration only allows spin-down resonant (1s-3d) excitations while a further oxidation of the Fe 3d shell will open up vacancies in the spin-up density of states. This is immediately detectable in spin-selective absorption spectroscopy which thus serves as a “smoking gun” technique to detect Fe(IV) species. Figure 8 shows the full 1s3p RIXS plane of Fe2O3 as an example for a 3d5 system and of Fe in SrFeO2.89 as an example for an Fe(IV) containing compound. The weak feature at 7114.5 eV incident and 7045 eV emitted energy is a signature of a 3d4 configuration in SrFeO2.89 (cf. Figure 9).
 |
|
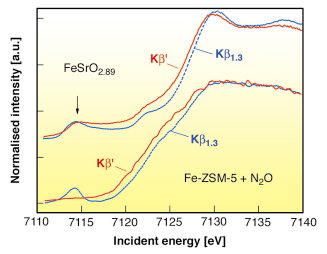
Fig. 9: XANES spectra of the Fe K-edge of FeSrO2.89 and of Fe-ZSM-5 after reaction with N2O (bottom), recorded on the Kß1,3 and the Kß’ fluorescence channel. The peak at 7114.5 eV incident energy in the Kß’ channel indicates a 3d4 configuration. |
Based on this proof of principle we applied 1s3p RIXS spectroscopy to Fe in a working catalyst. A Fe-ZSM-5 sample with an iron loading of 0.36 wt% was treated in steam at 873 K and finally in He at 1218 K. After this treatment, the majority of the iron sites should react with N2O either to the postulated Fe(IV) species or the oxidation process stops at Fe(III) species. Figure 9 compares the spin-selective absorption scans for SrFeO2.89 and Fe-ZSM-5 under N2O. We do not find any indication for a 3d4 configuration in Fe-ZSM-5 and thus reject the proposed formation of Fe(IV) species.
RIXS unequivocally proves that Fe(IV) species with a 3d4 spin configuration are not formed during the catalytic reaction of Fe-ZSM-5 with N2O. On the other hand, the partial 3d4 configuration of the perovskite FeSrO2.89 is clearly detected. The technique is applicable to all Fe containing systems where Fe(IV) systems are believed to exist and can be extended to some other 3d transition metal systems.
References
[1] A.E. Bocquet, A. Fujimori, T. Mizokawa, T. Saitoh, H. Namatame, S. Suga, N. Kimizuka, Y. Takeda, and M. Takano, Phys. Rev. B 45, 1561 (1992).
[2] L. Kiwi-Minsker, D.A. Bulushev, and A. Renken, J. Catal. 219, 273 (2003).
[3] G. Peng, F.M.F. Degroot, K. Hämäläinen, J.A. Moore, X. Wang, M.M. Grush, J.B. Hastings, D.P. Siddons, W.H. Armstrong, O.C. Mullins, and S.P. Cramer, J. Am. Chem. Soc. 116, 2914 (1994).
Principal Publication and Authors
G.D. Pirngruber (a), J.D. Grunwaldt (a), J.A. van Bokhoven (a), A. Kalytta (b), A. Reller(b), O.V. Safonova (c), and P. Glatzel (c), J. Phys. Chem. B 110, 18104 (2006).
(a) Eidgenössische Technische Hochschule, Zürich (Switzerland)
(b) University of Augsburg (Germany)
(c) ESRF



