- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2006
- Structural Biology
- X-ray radiation-induced damage in DNA monitored by online Raman spectroscopy
X-ray radiation-induced damage in DNA monitored by online Raman spectroscopy
The interaction of biological tissue with ionising radiation such as X-rays, electrons and ![]() -rays causes damage initiated by free radicals. Much effort has been invested in trying to understand the mechanisms involved, in particular for DNA, but these are still not well understood. It would therefore be of great interest if we could obtain new insights into the detailed structural consequences of early events of radiation damage to DNA.
-rays causes damage initiated by free radicals. Much effort has been invested in trying to understand the mechanisms involved, in particular for DNA, but these are still not well understood. It would therefore be of great interest if we could obtain new insights into the detailed structural consequences of early events of radiation damage to DNA.
Raman spectroscopy is a powerful technique capable of providing detailed information about the chemical nature of individual bonds. Due to the intricate molecular makeup of proteins and nucleic acids, the resulting Raman spectra are correspondingly complex. However, when the technique is adapted to crystals of biological macromolecules it offers distinct advantages compared to solution studies [1]: such crystals contain a 10 to 100 fold higher concentration of the macromolecule and have a relatively low solvent content. This improves the Raman signal-to-noise ratio substantially. Crystal packing forces, which reduce high-order movements, result in an increased resolution of Raman bands.
Recent technical advances in Raman spectroscopy include the advent of CCD detectors sensitive in the red-light region, thus avoiding luminescence at lower excitation wavelengths, and precision holographic notch filters to remove elastically-scattered photons. It is now possible to measure detailed backbone orientations, ring torsions and individual bond chemistry in nucleic acids. Here we employ Raman spectroscopy as a means to probe detailed chemical events during MX data collection.
A Raman spectrometer was adapted for use on a goniometer either at the cryobench laboratory [2] or on MX beamlines at the ESRF. Raman spectra of high-quality could be reproducibly collected from single DNA crystals kept at 100 K in a flow of cold nitrogen gas. Collection of non-resonant Raman spectra from native, single- and dibrominated DNA crystals allowed the unambiguous identification of the non-symmetric bending vibration for C-Br bonds in 8-bromo-2’-deoxyguanosine at 293 cm-1. Following these studies, Raman spectra were collected using the online setup at ID14-2 on a single dibrominated crystal before and after X-ray exposure. Debromination was clearly visible with the loss of the 293 cm-1 band corresponding to what was observed with MX at a similar dose (Figure 74). Comparison of spectra before and after exposure demonstrates multiple vibrational changes corresponding to specific X-ray induced damage sites in the DNA.
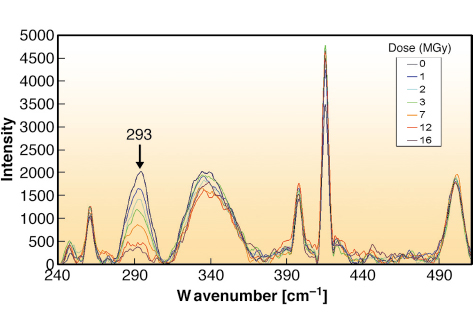 |
|
Fig. 74: Real-time Raman online monitoring of a dibrominated DNA crystal during continuous X-ray exposure at beamline ID14-2. Debromination can be followed using the decay of the 293 cm-1 band as a function of dose. |
A series of Raman spectra were collected whilst a stationary dibrominated DNA crystal was exposed to the X-ray beam. X-ray induced debromination was monitored in real-time as a function of dose. A decay constant of 7.1 MGy was obtained fitting the curve with a single exponential. Transient chemical changes could also be measured. A series of X-ray diffraction datasets were collected on a similar dibrominated DNA crystal on ID14-4. The decay of the electron density for the bromine atoms as a function of dose was analysed with the program SHARP. This allowed dose-dependant occupancy refinement which could also be fitted to a single exponential, correlating with the Raman studies.
Our initial online Raman studies have provided an independent observation of the susceptibility to radiation damage of halogenated nucleotides. We are now in the process of analysing the wealth of information available in the Raman spectra to further investigate the very fine details of the chemistry that takes place when X-rays ravage important (native) biomolecules.
References
[1] P.R. Carey, Annual Review of Physical Chemistry 57, 527-554 (2006).
[2] http://www.esrf.fr/UsersAndScience/Experiments/MX/Cryobench/Instruments/raman
Principal Publication and Authors
J.E. McGeehan (a), P. Carpentier (b), A. Royant (b), D. Bourgeois (b), and R.B.G. Ravelli (a), Journal of Synchrotron Radiation 14, 99-108 (2007).
(a) EMBL, Grenoble Outstation (France)
(b) Institut de Biologie Structurale, CNRS/CEA/UJF, Grenoble and ESRF (France)



