- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2006
- X-ray Imaging and Optics
- Cobalt puts uniform spin distributions into ZnO
Cobalt puts uniform spin distributions into ZnO
There is growing interest in the use of the spin degree of freedom in semiconductor quantum structures as a medium for the manipulation and storage of classical and quantum information. The confluence of both semiconductor and magnetic storage concepts is expected to have many advantages such as non-volatility, increased data processing speed, decreased electric power consumption, and increased integration densities compared to standard semiconductor devices. In all novel spin devices, diluted magnetic semiconductors (DMSs) play a key role. As one example, it has been suggested during the last few years that Co impurities in ZnO introduce exceptional magnetic properties, this may even lead to ferromagnetism with high Curie temperatures [1]. However, one of the key unanswered questions is whether the resulting material is indeed an alloy of (Zn,Co)O or whether it remains as ZnO with clusters, background impurities or second phases responsible for the magnetism. To clarify the mechanism by which ferromagnetism is produced, it is crucial to study the retention of single-phase elemental traces as well as local atomic distortions around Co sites, issues analysed in detail by the present work.
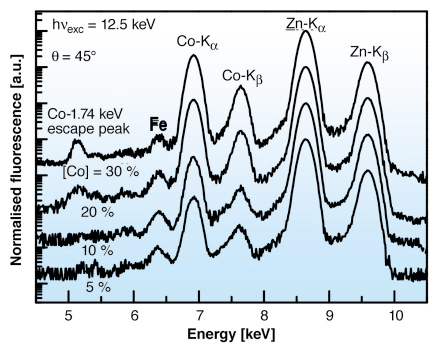
We have studied the Co incorporation on pulsed laser deposited (PLD) ZnO films with micrometre resolution at beamline ID22. Thin films were prepared at 400°C on sapphire substrates with a controlled atmosphere of 5N oxygen at 2 x 10-4 mbar. The monochromatic beam was focused using Kirkpatrick-Baez mirrors (at Co K-edge ~1010 photons/second in the focused beam: 1.5 x 3.5 µm2 size). The X-ray fluorescence (XRF) data are shown in Figure 135. Co concentrations, ranging from 5% up to about 26%, were estimated from the Co and Zn K![]() line intensity ratio [2]. The presence of residual Fe atoms could play a key role in the resulting magnetism. Elemental maps of Co and Zn were also obtained by measuring their K
line intensity ratio [2]. The presence of residual Fe atoms could play a key role in the resulting magnetism. Elemental maps of Co and Zn were also obtained by measuring their K![]() line intensities over the sample surface. Uniform patterns with no intensity changes (< 0.02%) were observed for all samples, showing a homogeneous distribution of both elements at the length scale of the beam size.
line intensities over the sample surface. Uniform patterns with no intensity changes (< 0.02%) were observed for all samples, showing a homogeneous distribution of both elements at the length scale of the beam size.
 |
|
Fig. 135: XRF spectra of ZnO samples with different Co content taken at 12.5 keV. |
XANES spectra around the Zn and Co K edges are shown in Figure 136a, shifted horizontally for clarity. Our results strongly suggest a substitutional Co incorporation into the Zn sites. A direct comparison to the XANES data around Co K edge for several model compounds confirmed predominant tetrahedral coordination of Co atoms in ZnO. Finally, Figure 136b shows the magnitude of the Fourier transforms of the Co K-edge EXAFS functions for low and high Co concentrated ZnO. Within experimental accuracy, there is no significant change of the first and second neighbour distances as a function of the Co content. The spectra look very similar, except for an apparent reduced magnitude of the nearest O-neighbour peak. This amplitude reduction is accounted for by the increase of the nearest O–neighbour Debye-Waller (DW) factor ![]() 2Co-O at higher Co concentration. The small decrease in the Co–Zn distance, on the other hand, might be attributed to the competing effects of the lattice parameter reduction induced by Co incorporation and the tensile strain due to the pseudomorphic growth on sapphire (lattice mismatch ~ 16%). The increase in the DW factor as a function of the Co concentration can be understood because the higher static disorder induced on the Co–Zn distance by the strong Co–O interaction.
2Co-O at higher Co concentration. The small decrease in the Co–Zn distance, on the other hand, might be attributed to the competing effects of the lattice parameter reduction induced by Co incorporation and the tensile strain due to the pseudomorphic growth on sapphire (lattice mismatch ~ 16%). The increase in the DW factor as a function of the Co concentration can be understood because the higher static disorder induced on the Co–Zn distance by the strong Co–O interaction.
 |
|
Fig. 136: a) Comparison of the Co and Zn K-edge XANES for low and high Co content in ZnO. b) Comparison of the Fourier transforms of the Co K-edge EXAFS functions. The dashed lines correspond to the data fits using FEFFIT program. |
In summary, the Co concentration was estimated for all ZnO samples by XRF analysis. XANES spectra around both Co and Zn K-edges retained their main characteristics with the Co content (i.e. the number and energy positions of the principal resonances), strongly indicating Co on substitutional Zn sites. Chemical analysis revealed predominant tetrahedral coordination geometry of Co atoms in ZnO. Within the resolution of the hard X-ray microprobe, uniform spatial distributions of Co and Zn sites were detected on the micrometre scale. Finally, the EXAFS results provided direct evidence for the substitution of Co atoms in Zn sites.
References
[1] T. Dietl, H. Ohno, F. Matsukura, J. Cibert, D. Ferrand, Science, 287, 1019-1022 (2000).
[2] G. Martinez-Criado, A. Somogyi, S. Ramos, J. Campo, R. Tucoulou, M. Salome, J. Susini, M. Hermann, M. Eickhoff, M. Stutzmann, Appl. Phys. Lett., 86, 131927 (2005).
Principal Publication and Authors
G. Martinez-Criado (a), A. Segura (b), J.A. Sans (b), A. Homs (a), J. Pellicer-Porres (b), J. Susini (a), Appl. Phys. Lett., 89, 061906 (2006).
(a) ESRF
(b) Applied Physics Department, Valencia University (Spain)



