- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2007
- Materials Science
- Phosphorous becomes six-fold-coordinated by oxygen under high pressure
Phosphorous becomes six-fold-coordinated by oxygen under high pressure
Aluminium phosphate is a quartz analogue that gives its name to a family of oxides called berlinites, which are characterised by spirals of tetrahedra like those of silica, but where Si is alternatively replaced by Al and P. The high-pressure behaviour of quartz-like materials has been the subject of many studies in the past because, apart from their intrinsic interest, their high-pressure behaviour can help towards understanding polymorphism in silica. Previous Raman and XRD measurements showed that the high-pressure phase of AlPO4 might be a poorly crystallised solid with lattice parameters corresponding to the InPO4 structure. The stability of this structure has been confirmed through ab initio calculations, although a discrepancy remained between the calculated and measured lattice parameters. In the InPO4 structure, the aluminium atoms are octahedrally bonded, whereas the phosphorus atoms remain in their tetrahedral coordination scheme.
At higher pressure, a denser structure is expected for berlinite, where both cations would be in an octahedral environment, as happens in GaAsO4 or AlAsO4. However, to the best of our knowledge, a pressure-induced change in coordination from 4 to 6 for a compound containing phosphorus has never been reported before.
We have performed angle-dispersive X-ray diffraction experiments up to the 100 GPa range at beamline ID09A. The laser heating technique was used at some of the pressure points to increase the kinetics of the transformation and also to improve the quality of the spectra. The experimental findings are supported and complemented by ab initio calculations.
 |
|
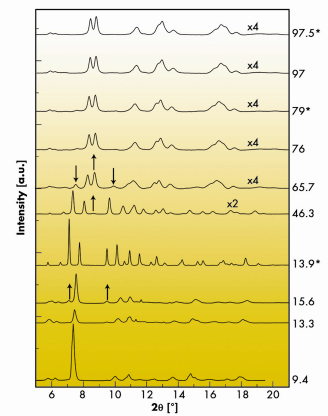
Fig. 35: X-ray diffraction spectra under high pressure. |
Figure 35 shows the experimental spectra. At 13.3 GPa new peaks not belonging to the berlinite phase appear, which become more evident at 15.6 GPa. At this point we annealed the sample with the laser heating system. As the spectrum shows, this produced a complete transition. The indexing supports an InPO4 type phase, which is confirmed by the Rietveld refinement.
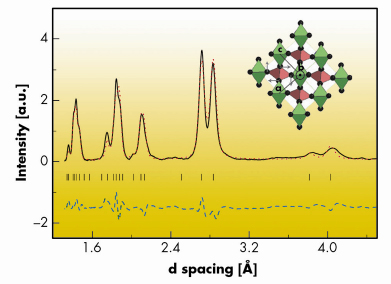
The InPO4 phase is stable up to 46.3 GPa. Between 46 and 66 GPa, AlPO4 transforms in a continuous way. At 76 GPa, most of the sample has adopted a new structure. Further pressure increase or laser heating has little impact on the spectra up to 97.5 GPa, the maximum pressure studied. At 97.5 GPa, the diffraction peaks can be indexed with a monoclinic cell. The indexing shows a striking similarity to that of orthorhombic CaCl2 silica. This similitude is translated to the equation of state. The Rietveld refinement is presented in Figure 36. We conclude that after the second phase transition AlPO4 adopts a monoclinic distorted CaCl2 structure (m-CaCl2), which is sketched in the inset of Figure 36. The ab initio computations support the experimental findings, as they show that the m-CaCl2 phase is stable with respect to the InPO4 phase from 31.5 GPa up, and also yield very close internal parameters and equations of state. The main structural novelty induced by the phase transition is the increment in phosphorus coordination from 4 to 6.
 |
|
Fig. 36: Rietveld refinement of the spectrum acquired at 97.5 GPa with a m-CaCl2 model. Inset: m-CaCl2 sketch. Green and red polyhedra are centred around Al and P atoms, respectively. |
The observed phase-transition sequence can be discussed in the context of previous studies, which shed light on the complex phase diagram of SiO2. According to these studies, high-pressure-induced densification is accompanied in a first step by the formation of a (distorted) hexagonal sublattice of oxygen atoms. In a second step, the smaller silicon cations redistribute in the interstices. In AlPO4 we observe a similar pattern and add the possibility, as in other berlinites, of a pressure-delayed change in coordination of the two cations. The hexagonal compact array of oxygens (ABC, slightly distorted) is already present in the InPO4 phase. Al occupies octahedral interstices but P is still in tetrahedral interstices. AlO6 octahedra form edge-sharing rows connected by PO4 tetrahedra. In the m-CaCl2 phase the oxygen stacking changes to ABAB... and P atoms modify their site and attain the octahedral configuration. Both cations now define non-kinked edge-sharing octahedral rows, connected by vertices.
Finally, the finding of phosphorous octahedrally coordinated by oxygen may open new routes in high-pressure phosphorous chemistry. The existence of numerous crustal silicates where Si is six-fold coordinated is well known. In particular, the rutile, hollandite and calcium ferrite structures are formed from edge-sharing chains of silicon octahedra. The observation of the m-CaCl2 structure in AlPO4 and the close relationship of AlPO4 with silica demonstrates the possibility of completely unexplored families of compounds analogous to those of the silicates but based on PO6.
Principal publication and authors
J. Pellicer-Porres (a), A.M. Saitta (b), A. Polian (b), J.P. Itié (c) and M. Hanfland (d), Nature Materials 6, 698-702 (2007).
(a) ICMUV, Universidad de Valencia, Burjassot (Spain) + Malta Consolider Team.
(b) Physique des Milieux Denses, IMPMC, CNRS, Université Pierre et Marie Curie -Paris 6 (France)
(c) Synchrotron SOLEIL, Gif-sur-Yvette (France)
(d) ESRF



