- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2007
- Structural Biology
- Crystal structure of FhaC: molecular insights into how proteins get across the outer membrane
Crystal structure of FhaC: molecular insights into how proteins get across the outer membrane
Targeting of proteins to their dedicated subcellular compartments is essential for cell function and organelle biogenesis. Translocation of proteins across, or insertion into membranes is mediated by protein machines, some of which have been conserved throughout evolution, such as the transporters of the Omp85/TpsB superfamily. TpsB transporters are components of “Two-Partner Secretion” (TPS) systems in Gram negative bacteria [1]. They secrete large, mostly ß-helical proteins called “TpsA” proteins that generally serve as virulence factors. TpsB transporters function as monomers and without accessory factors. The superfamily also includes the Toc75, Sam50/Tob55 and Omp85/YaeT homologs, which are the cores of large hetero-oligomeric complexes involved in protein transport across, and insertion of b-barrel proteins into, the outer membranes of chloroplasts, mitochondria and Gram-negative bacteria. In recent years, researchers have begun to make progress in understanding how these transporters work. The structure of FhaC, the outer-membrane transporter that secretes the Bordetella pertussis adhesin filamentous haemagglutinin (FHA) now provides structural insights into this process.
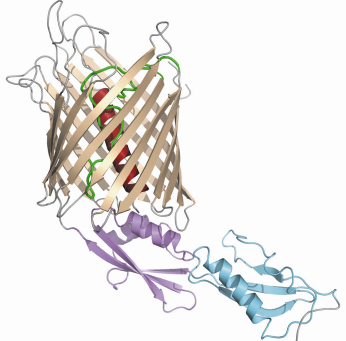
The FhaC structure was solved by the single-wavelength anomalous diffusion (SAD) method with diffraction data measured to 3.5 Å resolution on BM14, and subsequently refined to 3.15 Å resolution using data measured on ID14-4. The structure shows a ß barrel with an N-terminal extension consisting of an ![]() helix and two periplasmic POTRA domains, which are organised around a three-stranded ß sheet and one
helix and two periplasmic POTRA domains, which are organised around a three-stranded ß sheet and one ![]() helix (Figure 82). The ß barrel consists of 16 antiparallel ß strands connected by short turns at the periplasmic side and long loops at the cell surface. The channel within the barrel is occluded by loop L6, which folds into the barrel, and by the N-terminal
helix (Figure 82). The ß barrel consists of 16 antiparallel ß strands connected by short turns at the periplasmic side and long loops at the cell surface. The channel within the barrel is occluded by loop L6, which folds into the barrel, and by the N-terminal ![]() helix, which spans the channel interior. The observed residual opening is too narrow to allow for transport of a protein, even in an extended conformation. However, upon reconstitution of FhaC into planar lipid bilayers and application of a transmembrane potential, much wider channels were revealed, corresponding to channel widths of 8 to 10 Å. Thus, the channel appears to be dynamic: Upon binding of FHA to POTRA-1, the channel may open by extrusion of the
helix, which spans the channel interior. The observed residual opening is too narrow to allow for transport of a protein, even in an extended conformation. However, upon reconstitution of FhaC into planar lipid bilayers and application of a transmembrane potential, much wider channels were revealed, corresponding to channel widths of 8 to 10 Å. Thus, the channel appears to be dynamic: Upon binding of FHA to POTRA-1, the channel may open by extrusion of the ![]() helix and/or loop L6, thus creating a protein translocation pathway. Previous work indeed showed a topological rearrangement in L6 upon coexpression of FHA.
helix and/or loop L6, thus creating a protein translocation pathway. Previous work indeed showed a topological rearrangement in L6 upon coexpression of FHA.
 |
|
Fig. 82: Ribbon representation of FhaC viewed from the membrane plane. The |
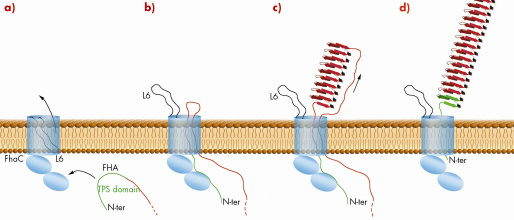
Collectively, the data allowed us to propose the following model for transport of FHA across the outer membrane (Figure 83). The N-terminal TPS domain of FHA, which folds as a ß helix after translocation [2], initially interacts in an extended conformation with the POTRA 1 domain in the periplasm. These interactions bring regions of FHA in proximity to the tip of loop L6 (Figure 83a). Conformational changes in FhaC then expel loop L6 out of the ß barrel, opening the channel for FHA translocation. FHA may form a hairpin made up of two extended polypeptide chains in the channel, with its TPS domain anchored in the periplasm (Figure 83b). The first repeats of the adhesin then reach the cell surface, where they progressively fold into ß-helical coils. The formation of the FHA rigid ß helix may provide the energy to drive its translocation through FhaC (Figure 83c). After the C-terminus of FHA has reached the surface, the TPS domain dissociates from the POTRA domains and is translocated, capping the N-terminus of the FHA ß helix. Finally, loop L6 can move back into the barrel (Figure 83d).
 |
|
Fig. 83: Proposed model of FHA transport across the outer membrane. |
The transport mechanism proposed here may apply more generally to the secretion of TpsA proteins by their dedicated TpsB transporters. All members of the Omp85/TpsB superfamily harbour one to several POTRA domains followed by a ß barrel, as well as conserved motifs corresponding to the L6 loop within the barrel, and they mostly handle substrate proteins rich in ß structure. Therefore, the major features described here are likely to remain valid throughout the family, although more complex molecular events are expected for some of these transporters given that they are part of macromolecular assemblies.
In conclusion, we have determined the first crystal structure of a member of the Omp85-TpsB transporter superfamily. It offers molecular insights into how proteins get into and across cellular membranes.
References
[1] J. Mazar, P.A. Cotter, Trends Microbiol 15, 508-15 (2007).
[2] B. Clantin, H. Hodak, E. Willery, C. Locht, F. Jacob-Dubuisson, V. Villeret. PNAS 101, 6194-9 (2004).
Principal publication and authors
B. Clantin (a), A.-S. Delattre (b), P. Rucktooa (a), N. Saint (c), A. Méli (c), C. Locht (b), F. Jacob-Dubuisson (b), V. Villeret (a), Science 317, 957-961 (2007).
(a) IBL UMR8161 CNRS, Lille (France)
(b) U629, Lille (France)
(c) U554, Montpellier (France)



