- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2009
- Structure of materials
- A miniaturised linear pH sensor based on a highly photoluminescent self-assembled europium(III) metal-organic framework
A miniaturised linear pH sensor based on a highly photoluminescent self-assembled europium(III) metal-organic framework
Research in the field of lanthanide-based metal-organic frameworks (Ln-MOFs) is growing rapidly because of the discovery of new structures with potential applications in catalysis, sensors, contrast agents, non-linear optics, display and electroluminescent devices. In particular, photoluminescence applications need high quantum efficiency to minimise energy losses and to allow miniaturisation.
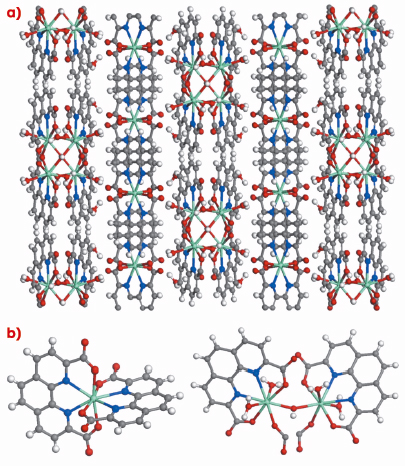
We focused our attention on 1,10-phenanthroline-2,9-dicarboxylic acid (H2PhenDCA) as a suitable organic ligand, since it is well known as a sensitising agent for Eu3+ and is able to coordinate Eu centres through the oxygen atoms of the carboxylate groups and the nitrogen of the phenanthroline. PhenDCA2- tends to form zero-dimensional (molecular) water soluble compounds, but we were hoping to obtain a 2D or 3D insoluble equivalent in order to transfer these properties to a solid, which could have practical applications. This was accomplished through a hydrothermal synthetic route, a powerful technique for the preparation of metastable compounds, via which it was possible to obtain a new material, named ITQMOF-3. Its crystal structure was solved using single crystal X-ray diffraction data collected at beamline BM16 (Figure 61).
 |
|
Fig. 61: a) Crystal structure of ITQMOF-3 showing the lamellar packing; b) Coordination environments of Eu1 (left) and Eu2 (right); C grey, N blue: H white, O red, Eu orange. |
ITQMOF-3 can be described as a layered compound with two distinct and very different sheets, A and B. Each sheet contains one type of Eu3+ ion (Eu1 or Eu2) in a different chemical environment. Sheet A, containing Eu1, has ML2 stoichiometry and is negatively charged. Each Eu3+ ion is octacoordinated by two tetradentate ligands to form isolated Eu(PhenDCA)2 species. The Eu(PhenDCA)2 groups interact with the neighbouring groups in the sheet through multiple ![]() -
-![]() interactions of the aromatic rings, producing a two-dimensional self-assembled layer. Sheet B contains Eu2 with stoichiometry [Eu2(PhenDCA)2(OH)–(OH2)4], and is thus positively charged. In this case, the sheet is formed by isolated planar chains along the c axis, with the OH– and half of the PhenDCA molecules acting as bridges between neighbouring Eu3+ ions. Consequently, Eu2 is also octacoordinated, though in a very different environment from that of Eu1, and is situated close to the neighbouring Eu3+ ions. The linkage between adjacent A and B sheets take place mainly through electrostatic interactions because of their respective negative and positive charges.
interactions of the aromatic rings, producing a two-dimensional self-assembled layer. Sheet B contains Eu2 with stoichiometry [Eu2(PhenDCA)2(OH)–(OH2)4], and is thus positively charged. In this case, the sheet is formed by isolated planar chains along the c axis, with the OH– and half of the PhenDCA molecules acting as bridges between neighbouring Eu3+ ions. Consequently, Eu2 is also octacoordinated, though in a very different environment from that of Eu1, and is situated close to the neighbouring Eu3+ ions. The linkage between adjacent A and B sheets take place mainly through electrostatic interactions because of their respective negative and positive charges.
ITQMOF-3 is insoluble in water, and possesses an excellent thermal stability in air to around 300ºC. Moreover, it is stable in aqueous solution in a pH range between around 4 and 8. The photoluminescence of ITQMOF-3 displays a very high absolute emission quantum yield, with the 5D0![]() 7F0 transition in the emission spectra presenting two separated peaks, corresponding to the Eu3+ ions in the Eu2 and Eu1 sites. In the case of Eu2, the position and intensity of this transition is dependent on variations of the coordination environment, which suggested its potential use as a sensor (Figure 62).
7F0 transition in the emission spectra presenting two separated peaks, corresponding to the Eu3+ ions in the Eu2 and Eu1 sites. In the case of Eu2, the position and intensity of this transition is dependent on variations of the coordination environment, which suggested its potential use as a sensor (Figure 62).
 |
|
Fig. 62: Intensity variation of the Eu2 5D0 |
Our preliminary results for the use of ITQMOF-3 as a pH sensor have been very encouraging. The intensity of the 5D0![]() 7F0 transition for Eu2 varies linearly and fully reversibly within the pH range in which the material is stable. Interestingly, this pH range corresponds to that required for working with biological fluids, such as blood and cell-culture media. The most outstanding property of this new material for pH sensing is that it requires no calibration because the intensity corresponding to Eu1 is not affected by pH, and can be used as an internal standard. In fact, the pH of a solution can be determined with high accuracy from the linear relationship between the relative Eu2/Eu1 emission intensities (Ir). Thus, by taking advantage of the main properties of ITQMOF-3-Eu (high emission quantum efficiency, insolubility in aqueous media, stability in a relatively wide range of pH, and pH-sensing capability) it has been possible to design a new miniaturised pH sensor prototype by combining the material with a commercial optical fibre. By using this device, we were able to measure continuously the pH in solution in the range 5-7.5 with good accuracy and a rapid response.
7F0 transition for Eu2 varies linearly and fully reversibly within the pH range in which the material is stable. Interestingly, this pH range corresponds to that required for working with biological fluids, such as blood and cell-culture media. The most outstanding property of this new material for pH sensing is that it requires no calibration because the intensity corresponding to Eu1 is not affected by pH, and can be used as an internal standard. In fact, the pH of a solution can be determined with high accuracy from the linear relationship between the relative Eu2/Eu1 emission intensities (Ir). Thus, by taking advantage of the main properties of ITQMOF-3-Eu (high emission quantum efficiency, insolubility in aqueous media, stability in a relatively wide range of pH, and pH-sensing capability) it has been possible to design a new miniaturised pH sensor prototype by combining the material with a commercial optical fibre. By using this device, we were able to measure continuously the pH in solution in the range 5-7.5 with good accuracy and a rapid response.
Principal publication and authors
B.V. Harbuzaru (a), A. Corma (a), F. Rey (a), J.L. Jordá (a), D. Ananias (b), L.D. Carlos (b) and J. Rocha (b), Angewandte Chemie International Edition 48, 6476-6479 (2009).
(a) Instituto de Tecnología Química, (UPV-CSIC), Valencia (Spain)
(b) Departments of Chemistry and Physics, CICECO, University of Aveiro (Portugal)



