- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2010
- Dynamics and extreme conditions
- Melting points under pressure: the end of a controversy
Melting points under pressure: the end of a controversy
In recent years, great effort has been devoted to the investigation of the melting curve of elements at high pressure. However, melting remains a phase transition difficult to predict even with the most advanced theoretical methods. On the experimental side, attaining and measuring pressures of a few millions of atmospheres and temperatures of several thousands of K, and at the same time, reaching and identifying the equilibrium state of the material is also a challenge.
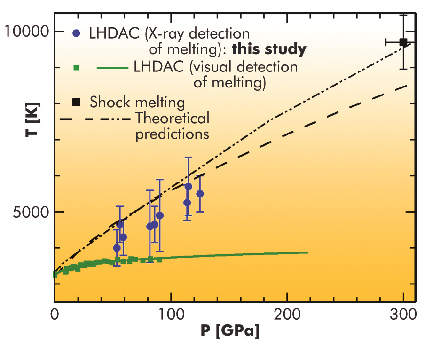
The melting curve of tantalum (Ta) exemplifies the efforts, problems and controversies in the determination of the melting curves of elements. The reported melting temperatures of tantalum were spread over several thousand degrees in the pressure range 100–300 GPa. Using shock compression, created by the impact of a projectile on the sample, a melting point of ~ 9700 K at 300 GPa has been determined. In laser-heated diamond anvil cells (LHDAC), where the sample is statically compressed between two diamonds and heated by a focussed infrared laser, melting points of ~ 3730 K at 100 GPa (obtained by visual detection) have been reported. This is only a few hundred K higher than the melting point at ambient pressure (3290 K), hence revealing a very flat melting curve as a function of pressure. Such a large discrepancy, outside the estimated error bars of the measurements, has stimulated many discussions. Various explanations have been proposed based on a possible difference in nature of the transformation seen by static and dynamic techniques [1].
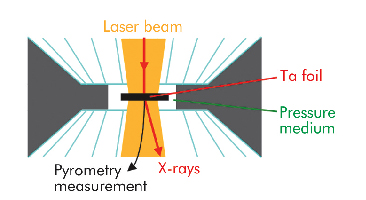
We have used a new methodology, based on X-ray diffraction, to detect the melting of tantalum in an LHDAC, building on a previous study of lead [2]. At beamline ID27, X-ray and laser beams were focussed on a common point within the sample while its temperature was measured by pyrometry (Figure 9). By recording the sample’s characteristic X-ray diffusion signal we were able to identify the state of the sample, including chemical reaction within it. The measurement of the solid sample specific volume further allowed the temperature path scenario provided by pyrometric measurements to be checked.
 |
|
Fig. 9: Schematic drawing of the pressure chamber in the laser heated diamond anvil cell. Various pressure media (alkali halides, rare gas solids, oxides) have been used. |
Our experiments showed that tantalum is chemically reactive in the LHDAC; in particular, it reacted with the diamond anvil to form a carbide. Such chemical reactions strongly perturb the observation of structural changes of the sample along a heating path. We also observed that the melting of the medium which surrounds the sample, called pressure medium, can affect pyrometry measurements because of a change of optical properties of the sample assembly and movements of the sample. This parasitic effect is a real problem for highly refractive materials such as Ta. A large fraction of our temperature measurements, biased by one of these effects, had to be discarded. The reliable data showed that the melting temperature of tantalum was much higher than previously measured in an LHDAC (for instance, 5500±500 K at 125 GPa). We furthermore observed that tantalum remained in the body-centred cubic phase up to the melting point.
 |
|
Fig. 10: Melting point determinations and predictions for tantalum. |
Our new LHDAC melting point results reconcile both the shock melting point and theoretical predictions (see Figure 10). We believe that this study has set a new standard for LHDAC studies; a similar approach, based on in situ X-ray diffraction, should be used for the measurement of the iron melting curve.
Principal publication and authors
A. Dewaele (a), M. Mezouar (b), N. Guignot (c), and P. Loubeyre (a), Phys. Rev. Lett. 104, 255701-255704 (2010).
(a) CEA de Bruyères le Châtel (France)
(b) ESRF (c) Synchrotron Soleil (France)
References
[1] C.J. Wu, P. Söderlind, J.N. Glosli and J.E. Klepeis, Nature Mater. 8, 223-228 (2009).
[2] A. Dewaele, M. Mezouar, N. Guignot and P. Loubeyre, Phys. Rev. B 76, 144106-144113 (2007).



