- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2010
- Dynamics and extreme conditions
- Pressure induced Fe ↔ Cu valence exchange and its structural consequences
Pressure induced Fe ↔ Cu valence exchange and its structural consequences
Diamond anvil cells (DAC) can be used to reach pressures beyond 100 GPa. They have allowed the elucidation of electronic phenomena such as intra d-d or inter p-d bands overlap leading to correlation breakdown (the Mott transition), spin-crossover due to crystal field enhancement, and variation of the magnetic ordering temperature. The interest in these pressure-induced electronic transitions became more momentous after recognising their occurrence with simultaneous structural phase transitions, caused by an electronic transition.
Here we report on the high pressure (HP) studies of a binary transition metal oxide, delafossite, which is composed of layers of Cu1+, Fe3+ and O2- ions (CuFeO2) forming a hexagonal structure with R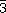 m symmetry (left inset in Figure 15). The triangular sub-lattices of antiferromagnetic Fe3+ moments are separated by layers of nonmagnetic Cu1+ and O2- along the c-axis. The weak Fe-O-Cu-O-Fe superexchange creates a 2D magnetic structure resulting in a spin-frustrated system. Our earlier studies revealed that only above ~18 GPa a 3D structure is established with long range magnetic ordering at TN = 40 K. This finding [1] motivated the present work for which the initial purpose was to search for the new structure that undergoes the 2D>3D transition.
m symmetry (left inset in Figure 15). The triangular sub-lattices of antiferromagnetic Fe3+ moments are separated by layers of nonmagnetic Cu1+ and O2- along the c-axis. The weak Fe-O-Cu-O-Fe superexchange creates a 2D magnetic structure resulting in a spin-frustrated system. Our earlier studies revealed that only above ~18 GPa a 3D structure is established with long range magnetic ordering at TN = 40 K. This finding [1] motivated the present work for which the initial purpose was to search for the new structure that undergoes the 2D>3D transition.
 |
|
Fig. 15: Pressure dependence of the unit-cell volume and crystal anisotropy reflected by the c/a ratio at room temperature. Circles correspond to the R3m phase, squares to the C2/c, and triangles to the P3m structures. The open points correspond to data recorded at decompression. The discontinuous decrease in c/a and V occurs at the onset of the HP1 phase. |
The methods used for this study were 57Fe Mössbauer spectroscopy, X-ray diffraction, and X-ray absorption spectroscopy (XAS), the latter two carried out at beamlines ID09A and ID24. XAS studies were performed both at the Fe and Cu K-edge using perforated anvils.
The sturdy O-Cu-O dumbbell backbones with orientation parallel to the c-axis, are responsible for the unusual robustness of the anisotropic low pressure region (0-18 GPa). With increasing pressure c/a increases until the R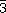 m structure becomes unstable and a first-order transition takes place, accompanied by reversal of the c/a slope and a discontinuous decrease in V(P) (HP1 range in Figure 15). In the new C2/c structure the O-Cu-O axis are tilted by 28° with respect to the c-axis. This signals the offsetof the 2D nature of delafossite and allows the onset of magnetic ordering at 40 K; a typical example of a structurally induced electronic/magnetic transition.
m structure becomes unstable and a first-order transition takes place, accompanied by reversal of the c/a slope and a discontinuous decrease in V(P) (HP1 range in Figure 15). In the new C2/c structure the O-Cu-O axis are tilted by 28° with respect to the c-axis. This signals the offsetof the 2D nature of delafossite and allows the onset of magnetic ordering at 40 K; a typical example of a structurally induced electronic/magnetic transition.
 |
|
Fig. 16: Mössbauer spectra of CuFeO2 recorded at 19 and 27 GPa. The inset summarises the TN(P) of the two Fe species, Fe3+ and Fe2+ (PM - paramagnetic; AF - antiferromagnetic). |
With further pressure increase, we made an astounding observation. Mössbauer spectroscopy studies at P > 20 GPa uncovered a new phenomenon not observed hitherto, a pressure-induced reduction of Fe3+, e.g. Fe3+ (S = 5/2)  Fe2+ (S = 2). This dramatic occurrence can be seen in Figure 16. Up to ~ 23 GPa, within the C2/c stability field, Mössbauer spectra at T <<< TN (40 K) revealed a single hyperfine field characteristic of a six-folded ferric oxide. At P > 23 GPa a new spectral component (red curve) appeared. Its hyperfine interaction parameters, hyperfine field and isomer shift are typical of an Fe2+-O species. This new genus carried with it robust magnetic features: a threefold jump in TN (see inset in Figure 16) that can be attributed only to new, enhanced, superexchange interactions.
Fe2+ (S = 2). This dramatic occurrence can be seen in Figure 16. Up to ~ 23 GPa, within the C2/c stability field, Mössbauer spectra at T <<< TN (40 K) revealed a single hyperfine field characteristic of a six-folded ferric oxide. At P > 23 GPa a new spectral component (red curve) appeared. Its hyperfine interaction parameters, hyperfine field and isomer shift are typical of an Fe2+-O species. This new genus carried with it robust magnetic features: a threefold jump in TN (see inset in Figure 16) that can be attributed only to new, enhanced, superexchange interactions.
The most reasonable mechanism for the Fe3+ → Fe2+ process is the simultaneous onset of a Cu1+ → Cu2+ valence change resulting from direct or indirect Fe-Cu d-band overlap.
The K-edge Cu and Fe XAS studies clearly confirmed this conjecture: the Cu-K-edge absorption edge shifts by about 1 eV in the 20-27 GPa range, while the iron edge shows a similar, however, negative shift. Given that within the family of pure iron (copper) oxides the absorption edge is observed to increase with increasing valence, our XAS results are consistent with the discussed valence transformations.
The creation of a Cu2+ - O bonding leads not only to the onset of a new S=1/2 magnetic sublattice but also to an increase in the copper coordination number, from 2 to 4. The 4-coordinated Cu2+ species cannot be accommodated within the C2/c framework, and as a result, domains of the P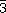 m structure appear, characterised by the formation of tetrahedral CuO4 distorted along the c-direction (HP2 range Figure 15). The XANES Cu simulation is in full accordance with the two Cu species valences and coordination numbers.
m structure appear, characterised by the formation of tetrahedral CuO4 distorted along the c-direction (HP2 range Figure 15). The XANES Cu simulation is in full accordance with the two Cu species valences and coordination numbers.
Epilogue: The HP studies of CuFeO2 concludes with the formation of a new super-structure at P > 23 GPa composed of a sub-structure Cu1+Fe3+O2 with space group C2/c and Cu2+Fe2+O2 with space group P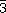 m. Their relative abundance at 29 GPa is 2:1 and their TN’s are 100 K and 300 K, respectively. The whole pressure process is reversible with no noticeable hysteresis.
m. Their relative abundance at 29 GPa is 2:1 and their TN’s are 100 K and 300 K, respectively. The whole pressure process is reversible with no noticeable hysteresis.
Principal publication and authors
W.M. Xu (a), G.Kh. Rozenberg (a), M.P. Pasternak (a), M. Kertzer (a), A. Kurnosov (b), L.S. Dubrovinsky (b), S. Pascarelli (c), M. Munoz (c), M. Vaccari (c), M. Hanfland (c), and R. Jeanloz (d). Phys. Rev. B 81, 104110 (2010).
(a) School of Physics and Astronomy, Tel Aviv University (Israel)
(b) Bayerisches Geoinstitut, University Bayreuth (Germany)
(c) ESRF
(d) Department of Earth and Planetary Science, University of California, Berkeley, CA (USA)
References
[1] W.M. Xu, M.P. Pasternak, and R.D. Taylor, Phys. Rev. B 69, 052401 (2004).



