- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2010
- X-ray imaging
- Functionalised nanoparticles in human cells by X-ray nano-imaging
Functionalised nanoparticles in human cells by X-ray nano-imaging
Biomedical applications of gold have a long history stretching back almost five thousand years. The dawn of the ‘nano-age’ has further broadened the potential of gold and other precious metals in medical applications, and today gold and platinum nanoparticles are being employed in entirely novel ways for improved diagnostics and therapy. In cancer treatment there is a delicate balance between attacking and killing the cancer cells and minimising harm to non-cancerous tissues. To minimise or even eliminate this “collateral” damage, the development of new nanodevices where one nanoparticle can specifically deliver therapeutic payloads, including small drug molecules or large biomolecules, to the targeted cancer cell, have become increasingly appealing for the future treatment of cancer. Gold and platinum nanoparticles have recently emerged as attractive candidates for delivery of therapeutic payloads [1]. The noble metal cores are chemically inert and non-toxic and therapeutic payloads including small drug molecules and/or large biomolecules (e.g. plasmid DNA or proteins for gene and protein therapy and targeting applications) can be attached via thiol linkages yielding stable nanovectors. For these reasons we are actively pursuing development of nanovectors with multiple functionalities. For many intended therapeutic applications, very few nanovectors delivered to the targeted cells and/or cellular compartment (e.g. cell nucleus), might be sufficient to achieve the desired effect. It is unlikely that any targeting strategy will be 100% target specific. This will result in at least some nanoparticles being internalised by healthy, non-targeted cells in low numbers. The imaging and detection of such low presence in cells represents a difficult problem and visualising where exactly the nanovectors go and in what numbers is extremely important.
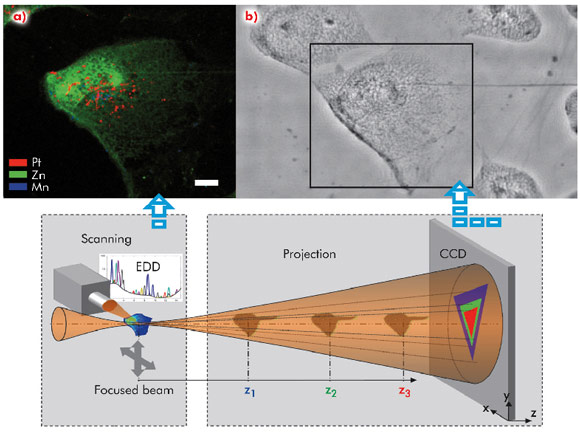
We have developed a new highly-sensitive synchrotron-based X-ray fluorescence microscopy (SR-XFM) technique to image the intracellular distribution of internalised noble metal nanoparticles.
SR-XFM has sufficient sensitivity to detect a single nanoparticle in the cell (few thousand atoms) in cryofixed and lyophilised cells. The nano-imaging end-station ID22NI with spatial resolution of 60 nm allows elemental imaging of whole cells (Figure 124). To mimic the targeted nuclear delivery, the CeL gold nanoparticles (CeL-AuNP) and CeL-platinum nanoparticles (CeL-PtNP) were transfected to MRC5VA normal human cells. The fluorescence map panel (Figure 124a) of the selected area from the relative phase map (Figure 124b) shows intracellular distribution of CeL-PtNP (with average diameter of 6 nm) with clear localisation predominantly in the nucleus (red pixels). Although no absorption contrast can be obtained from cells at the incident energy used, a relative phase map of a complete cell was acquired in the hard X-ray regime through projection microscopy using the partial coherence of the X-ray beam. The numerical phase retrieval procedure we have developed shows that a quantitative phase map with high spatial resolution and high sensitivity can be obtained highlighting very thin cellular structures (Figure 124b). This provides essential microstructural information from which the nucleus can be easily located and corroborates with the most intense zinc X-ray fluorescence signal (Figure 124a). Our new approach makes it possible to merge the information that will potentially provide a means to determine cellular volume and consequently an improved chemical quantification of intracellular metal-based nanovectors concomitantly to the level and sub-cellular distribution of physiologically essential metals. The nanofocus X-ray beam allows us to achieve detailed elemental maps with platinum single pixel hotspots likely to represent very small clusters of nanoparticles. Similarly, the technique proved to be very successful in intracellular localisation of CeL-gold nanoparticles in relation to other elements.
In summary, we have demonstrated the unprecedented potential of SR-XFM nano-imaging for the development of new nanoparticles as platforms for bioimaging and biodelivery applications. This technique has the potential to revolutionise the development of targeted nanoparticles.
Principal publication and authors
D.J. Lewis (a), C. Bruce (b), S. Bohic (c,d), P. Cloetens (d), S.P. Hammond (a), D. Arbon (b), S. Blair-Reid (b), Z. Pikramenou (a) and B. Kysela (b), Nanomedicine 5, 1547-1557 (2010).
(a) School of Chemistry, University of Birmingham (UK)
(b) School of Clinical and Experimental Medicine, University of Birmingham (UK)
(c) Institut des Neurosciences Grenoble (GIN), Université Joseph Fourier, Grenoble (France)
(d) ESRF
References
[1] P. Ghosh, G. Han, M. De, C.K. Kim and V.M. Rotello, Adv. Drug Deliv. Rev. 60, 1307-1315 (2008).