- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2010
- X-ray imaging
- Cellular insight into the human cerebellum by X-ray grating interferometry
Cellular insight into the human cerebellum by X-ray grating interferometry
The human brain has a complicated and fascinating micro-structure that requires adequate imaging modes. The established techniques of magnetic resonance imaging (MRI) and X-ray computed tomography (CT) are each subject to limitations. Whereas MRI provides superb contrast between the different components of brain tissue, such as white and grey matter, the spatial resolution of this technique prevents investigations on the cellular level. Whereas, conventional absorption micro-CT has the required spatial resolution but its contrast is limited for soft tissues.
A promising alternative is X-ray phase contrast microtomography, which is known to give superior contrast for light materials. Here we report on phase contrast tomography obtained by X-ray grating interferometry [1,2], a technique originally developed by researchers of the Paul Scherrer Institut, Switzerland, with the ESRF involved since the beginning. We applied the method in a comparative study of synchrotron-based absorption and phase microtomography of the human cerebellum.
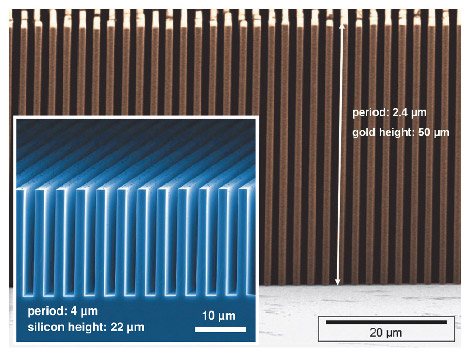
Phase tomography as used here yields the real part of the X-ray refractive index. This quantity is essentially proportional to the three-dimensional electron density distribution of the object. Contrast in the radiographs is generated by refraction of the X-ray beam in the specimen. In X-ray grating interferometry, the refraction signal is detected through an arrangement of deep transmission grid structures with micrometre-sized pitch, made using dedicated microstructuring processes (Figure 125). The gratings modulate the phase and/or amplitude profile of the X-ray wavefront.
The interferometric tomography of a human cerebellum was carried out at beamline ID19 with 23-keV X-rays. The interferometer consisted of a silicon phase grating with a line width of 2.4 µm and a structure height of 29 µm and an absorption grating with gold lines of width 1.2 µm (grating pitch 2.4 µm) and a structure height of 50 µm – i.e. an aspect ratio of more than 40. The two gratings were placed between the specimen and the detector, and separated by a propagation space of 480 mm length. During the measurements, the cerebellum, fixed in formalin, was immersed in a water tank with parallel acrylic glass plates.
 |
|
Fig. 125: Deep microstructured transmission gratings used in X-ray grating interferometers. Main picture: gold absorption grating made at KIT; Inset: silicon phase grating made at PSI. |
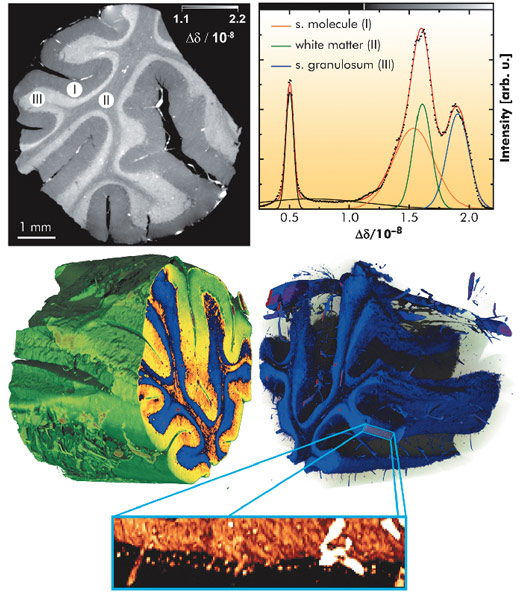
The reconstructed tomograms (Figure 126, top left) show distinct contrast between three different layers: stratum moleculare (I) and stratum granulosum (III), which both belong to grey matter, plus the white matter (II). Using a multi-Gaussian fit to the corresponding histogram (Figure 126, top right), we quantified the value of refractive index relative to the immersion medium (water), Δδ(x,y,z). The standard deviation of the retrieved values of beam deflection angle a in the unstructured background around the object was σα = 17 nrad. This value is a figure of merit for the measurement sensitivity of the refraction angle. The precision of the refractive index in the tomograms was identified by the width of the water peak in the histogram, σδ(H2O) = 2.3 x 10-10. This corresponds to an electron density resolution of 0.15 electrons per nm3 and a mass density sensitivity of 0.25 mg cm-3.
With a voxel size of 5 µm, the spatial resolution of the data is good enough to discriminate individual cells in the volume data. Thus, the dot-like structures near the bottom of the inset in Figure 126 are believed to be Purkinje cells, a type of large neuron in the brain. The tissue surrounding the cells has been made transparent in this 3D rendering.
These results clearly demonstrate that the spatial resolution and the sensitivity of this method are well suited for the discrimination between soft tissues such as white and grey matter and even for the visualisation of individual cells surrounded by tissue, without the use of any contrast agent.
Principal publication and authors
G. Schulz (a), T. Weitkamp (b), I. Zanette (b), F. Pfeiffer (c), F. Beckmann (d), C. David (e), S. Rutishauser (e), E. Reznikova (f) and B. Müller (a), J. R. Soc. Interface 7, 1665-1676 (2010).
(a) Biomaterials Science Center, University of Basel (Switzerland)
(b) ESRF
(c) Department of Physics/Biophysics (E17), Technische Universität München, Garching (Germany)
(d) Institute for Materials Research, Helmholtz-Zentrum Geesthacht (Germany)
(e) Laboratory for Micro- and Nanotechnology, Paul Scherrer Institut, Villigen (Switzerland)
(f) Institute for Microstructure Technology, Karlsruhe Institute for Technology (Germany)
References
[1] T. Weitkamp, A. Diaz, C. David, F. Pfeiffer, M. Stampanoni, P. Cloetens and E. Ziegler, Opt. Expr. 13, 6296–6304 (2005).
[2] F. Pfeiffer, O. Bunk, C. David, M. Bech, G. Le Duc, A. Bravin and P. Cloetens, Phys. Med. Biol. 52, 6923–6930 (2007).